The TriVersa NanoMate® LESA® is the latest in chip-based electrospray ionization technology from Advion Interchim Scientific®. It combines the benefits of liquid chromatography, mass spectrometry, chip-based infusion, fraction collection and direct surface analysis into one integrated ion source platform. It allows scientists to obtain more information from complex samples than LC/MS alone.
Liquid Extraction Surface Analysis Mass Spectrometry Method for Identifying the Presence and Severity of Nonalcoholic Fatty Liver Disease
The early stages of nonalcoholic fatty liver disease (NAFLD) are characterized by the accumulation of fat in the liver (steatosis). This can lead to cell injury and inflammation resulting in nonalcoholic steatohepatitis (NASH). To determine whether lipid profiling of liver tissue can identify metabolic signatures associated with disease presence and severity, we explored liquid extraction surface analysis mass spectrometry (LESA−MS, Advion TriVersa NanoMate with LESA PLUS) as a novel sampling tool. Using LESA−MS, lipids were extracted directly from the surface of ultrathin slices of liver tissue prior to detection by high-resolution mass spectrometry (MS).
We compared the data obtained by LESA− MS to that from liquid chromatography (LC)− MS and matrix-assisted laser desorption ionization MS. Advantages of LESA− MS include rapid analysis, minimal sample preparation, and high lipid coverage.
Furthermore, since tissue slices are routinely used for diagnostics in clinical settings, LESA− MS is ideally placed to complement traditional histology. Overall LESA− MS is found to be a robust, fast, and discriminating approach for determining NAFLD presence and severity in clinical samples.
Shotgun Lipidomics Analysis
Infusion based lipidomics analysis (shotgun Lipidomics) is an analysis strategy that involves the liquid extraction of lipids from samples such as blood, tissue homogenates or dried blood spots. This approach is suitable for a wide range of biomedical questions of lipid differentiation and lipid flux in the field of metabolic disease, neurological disorders, cancers or eye diseases. See how utilizing the Advion Interchim Scientific TriVersa NanoMate® can be used for high throughput shotgun Lipidomics analysis.
Myc Expression Drives Aberrant Lipid Metabolism in Lung Cancer
Published by the National Center for Biotechnology Information
MYC-mediated pathogenesis in lung cancer continues to attract interest for new therapeutic strategies. In this study, we describe a transgenic mouse model of KRAS-driven lung adenocarcinoma that affords reversible activation of MYC, used here as a tool for lipidomic profiling of MYC-dependent lung tumors formed in this model. Advanced mass spectrometric imaging and surface analysis techniques were used to characterize the spatial and temporal changes in lipid composition in lung tissue. We found that normal lung tissue was characterized predominantly by saturated phosphatidylcholines and phosphatidylglycerols, which are major lipid components of pulmonary surfactant. In contrast, tumor tissues displayed an increase in phosphatidylinositols and arachidonate-containing phospholipids that can serve as signaling precursors.
Lipid Species from Brain Tissue Sections Using LESA PLUS Liquid Extraction Surface Analysis PLUS LC Separation
Spatial lipid composition, distribution and regulation are very important factors for mediating lipid functionality and, when disrupted, can cause pathophysiological processes leading to cancer, obesity, atherosclerosis, and neurodegeneration. The novel LESAPLUS surface analysis approach combines the standard liquid extraction surface analysis with an additional step of a nano liquid chromatography separation. This combination is ideally suited to investigate small molecule drugs, metabolites or lipids from thin tissue sections.
LESA plus: Liquid Extraction Surface Analysis PLUS LC Separation
The Liquid Extraction Surface Analysis (LESA) capability of the TriVersa NanoMate enables simple, direct nanoESI mass spectrometric analysis from a variety of surfaces.
The new LESAPLUS allows for automated LESA experiments plus additional nano-LC separation through the ChipSoftX operating software with Developers Kit. This enhancement is ideal for direct tissue analysis.
CHIPSOFT 10.0 WITH DEVELOPERS KIT: Customized method development for the TriVersa Nanomate
ChipSoftX is an entirely new operating software for the TriVersa NanoMate automated nanoelectrospray source. Besides improvement in program compatibility with Windows and integration of existing software features, it also provides access to the new Developers Kit – a platform for customized method development with direct access to robot controls allowing entirely novel analysis workflows such as LESAPLUS.
Liquid extraction surface analysis field asymmetric waveform ion mobility spectrometry mass spectrometry for the analysis of dried blood spots
Analyst, 2015, Accepted Manuscript DOI: 10.1039/C5AN00933B
Liquid extraction surface analysis (LESA) is a surface sampling technique that allows electrospray mass spectrometry analysis of a wide range of analytes directly from biological substrates. Here, we present LESA mass spectrometry coupled with high field asymmetric waveform ion mobility spectrometry (FAIMS) for the analysis of dried blood spots on filter paper. Incorporation of FAIMS in the workflow enables gas-phase separation of lipid and protein molecular classes, enabling analysis of both haemoglobin and a range of lipid (phosphatidylcholine or phosphatidylethanolamine, and sphingomyelin species) from a single extraction sample. The work has implications for multiplexed clinical assays of multiple analytes.
Quantitative spatial analysis of the mouse brain lipidome by pressurized liquid extraction surface analysis (PLESA)
Reinaldo Almeida, Zane Berzina, Eva C. Arnspang, Jan Baumgart, Johannes Vogt, Robert Nitsch, and Christer S. Ejsing
Here we describe a novel surface sampling technique termed pressurized liquid extraction surface analysis (PLESA), which in combination with a dedicated high resolution shotgun lipidomics routine enables both quantification and in-depth structural characterization of molecular lipid species extracted directly from tissue sections.
University of Southern Denmark, Ejsing Lab
What is the focus of your lab’s research?
Development and applications of lipidomic technology that allow global analysis of cellular lipidomes with the ultimate goal of improving the understanding of the regulation of lipid metabolism.
Why did you incorporate the TriVersa NanoMate?
I used the TriVersa NanoMate in Andrej Schevchenko’s laboratory at Max Planck Institute in Dresden. I learned that the instrument was far easier to use than nanoelectrospray needles. Additionally, the TriVersa NanoMate o ffers high throughput and is easy to teach others, especially students, how to use. You can rely on the TriVersa NanoMate to get the job done.
Who would you recommend to purchase the TriVersa NanoMate?
The TriVersa NanoMate is essential for any laboratory interested in lipidomics research for its ease of use, reliability and stable ion spray.
Do you have any publications or presentations using the TriVersa NanoMate?
Publication highlight
Lipid Molecular Timeline Profiling Reveals Diurnal Crosstalk between the Liver and Circulation
Sprenger et al. Cell Rep. 2021 34(5):108710
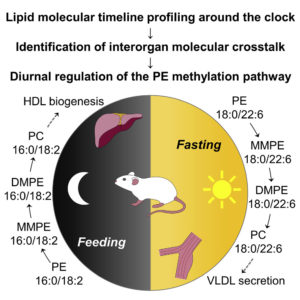
In-depth lipidomics and time-series analysis of fasting-feeding cycles and circadian rhythms provides insights into metabolic crosstalk between tissues.
Other Publications:
- Höring et al. Accurate quantification of lipid species affected by isobaric overlap in Fourier-transform mass spectrometry. Journal of Lipid Research. DOI: 10.1016/j.jlr.2021.100050
- Höring et al. Quantification of cholesterol and cholesterol ester by direct flow injection high-resolution fourier transform mass spectrometry utilizing species-specific response factors. Analytical Chemistry. DOI: 10.1021/acs.analchem.8b05013
- Artibani et al. Adipocyte-like signature in ovarian cancer minimal residual disease identifies metabolic vulnerabilities of tumor initiating cells. JCI Insight. DOI: 10.1172/jci.insight.147929
- Freyer et al. MIGA2 links mitochondria, the ER, and lipid droplets and promotes de novo lipogenesis in adipocytes. Mol Cell. DOI: 10.1016/j.molcel.2019.09.011
- Martinez-Montanes et al. Phosphoproteomic Analysis across the Yeast Life Cycle Reveals Control of Faty Acyl Chain Length by Phosphorylation of the Fatty Acid Synthase Complex. Cell Reports. DOI: 10.1016/j.celrep.2020.108024

