Blood Analysis using the Advion Interchim Scientific SOLATION® ICP-MS
Introduction
Trace elements are essential for proper biological functions in humans, and differing levels of trace elements are indicative of many diseases and conditions. Non-essential trace elements are also present in the human body as a result of environmental contaminants generated by human or industrial activities deposited in soil, air, water, and foodstuffs. These essential and non-essential trace elements are easily measured and monitored in blood, serum, and urine using ICP-MS.
In this application note we present a fast method for routine analysis of small volume blood samples for key toxic and essential elements using the SOLATION® Inductively Coupled Plasma Mass Spectrometer (ICP-MS) using a simple “dilute and shoot” sample preparation. The high sensitivity and wide dynamic range of ICP-MS are particularly important for the determination of trace levels of heavy metals, while simultaneously measuring nutritionally relevant elements at higher levels. Blood is a complex matrix, rich in proteins and salts that favor the formation of carbon- and chlorine-based interferences that affect many of the analytes. The collision cell on Advion Interchim Scientific’s SOLATION® ICP-MS is necessary for overcoming those interferences.
The SOLATION® ICP-MS has an octupole collision cell that is used for addressing interferences from polyatomic ions, especially for the transition metal elements. It is critical for robust and routine trace element analysis that the octupole cell does not become contaminated which could cause drift and unnecessary downtime. Ions passing through the interface are directed through a 90˚ turn and focused onto the entrance of the octupole using a quadrupole deflector (QD). Light and neutral particles continue through the QD and away from the cell.
The collision cell in the SOLATION® ICP-MS can be operated in “He Gas” mode in which the cell is filled with He to act as a collision gas, or in “No Gas” mode in which the cell is empty. The “He Gas” mode is used for isotopes subject to polyatomic interferences while the “No Gas” mode is used for the rest of the isotopes. The rapid switching between “He Gas” and “No Gas” modes on the SOLATION® (< 5 sec) ensures that analytical runs can be kept short, thereby improving productivity.

Experiment & Results
Reagents & Materials
Nitric acid (Aristar Plus, trace metal grade)
Triton X-100 (especially purified, Roche chemical)
Water, type 1 (18.2 MΩ, Elga point of use system)
Methanol (hypergrade for LC-MS, Supelco)
Mg, Ca, Mn, Cu, As, Se, Cd, Pb, Ge, Rh, Au, and Ir standard solutions (1000 μg/ml, Claritas ppt grade)
Trace Elements Whole Blood L-1 (SRM1) (Seronorm)
Trace Elements Whole Blood L-2 (SRM2) (Seronorm)
Trace Elements Whole Blood L-3 (SRM3) (Seronorm)
Blank Whole Blood (WB(F)) (UTAK)
Acid diluent: (0.5% Nitric acid, 0.05% Triton X-100, 2% Methanol, 0.25 μg/mL (ppm) Au, and the internal standards: 10 μg/L (ppb) Ge, Rh, and Ir)
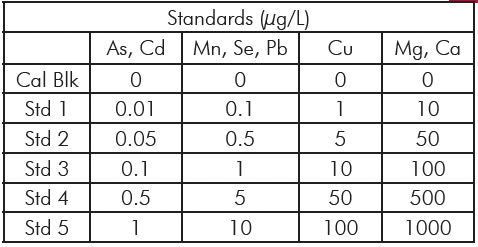
Table 1: Calibration and standard concentrations
Standards
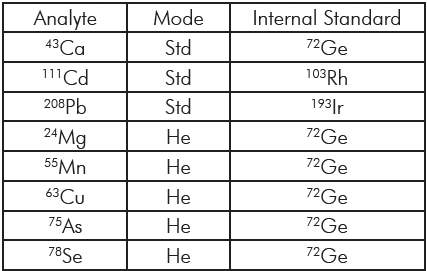
The method was developed to determine Mg, Ca, Mn, Cu, As, Se, Cd, and Pb in whole blood samples. The elements were chosen to represent some of the commonly measured metals in blood, covering both essential and toxic elements. Standards were prepared using the acid diluent with elements at four concentration levels to cover the range typically seen in blood. Table 1 outlines the standard concentrations at each level and which elements are at that level. The analysis mode and the internal standard used for each analyte are in Table 2. The ICH guidelines call for a minimum of five concentrations to establish linearity; with the calibration blank we use six, satisfying this requirement.
Table 2: Analysis Mode and Internal Standards

Samples & Preparation
Samples were prepared in 15mL, metal-free centrifuge tubes. Acid diluent (14.7mL) was added to each tube, followed by 0.3mL blood sample for a 1:50 dilution. The tubes were capped and inverted 3-5 times to thoroughly mix.
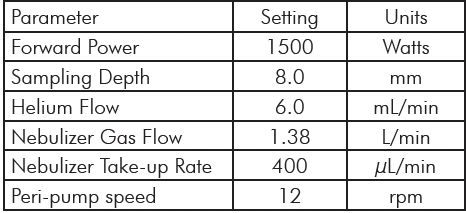
The samples were analyzed using a SOLATION® ICP-MS. The SOLATION® instrument configuration for this analysis was a cyclonic spray chamber with a Micromist concentric nebulizer and a one-piece torch. Ni sampler and skimmer cones were used throughout the study. The running conditions for the instrument are summarized in Table 3.
Table 3: ICP-MS Operating Parameters
Results & Discussion
We followed the ICH “Validation of Analytical Procedures” guidelines for method validation which defines specific requirements for accuracy, precision (repeatability), detection limit and method detection limit (DL and MDL), and quantitation limits (LOQ).
The accuracy of the method was established using the Seronorm reference materials, which had certified concentration values for all the elements in the method. These materials were measured in triplicate and the analytical results were compared to the certified values and 95% confidence limits provided by Seronorm.
These values are plotted on Figure 1 where the minimum and maximum certified values are represented as a box. Our analytical values are plotted in Figure 1, and in every instance, our values lie inside the limits indicating a high degree of accuracy which easily meets the ICH specifications.
A second measure of accuracy is spike recovery. The “blank blood” (WB(F)) sample from UTAK was spiked with 150μL of the stock standard solution for all elements except Ca and Mg in the 15mL tube. The recovery is calculated as:
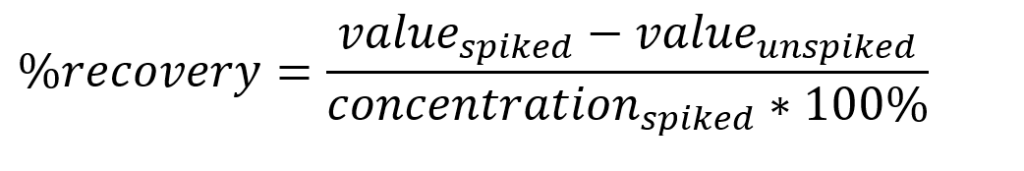
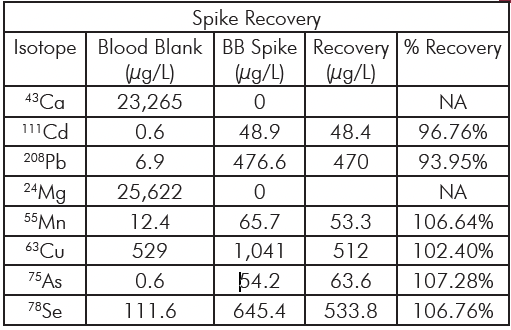
Spike recoveries are shown in Table 4. All recoveries are within 90 – 110%, indicating excellent recovery of the spiked analytes.
Table 4: Spike Recoveries
Method precision is measured as the repeatability of six Seronorm level 2 (SRM2) samples. Six samples of Seronorm level 2 (SRM2) were prepared, diluted, and analyzed individually. The results show less than 3% RSD among the replicates. The %RSD of the six replicates is presented in Figure 2.
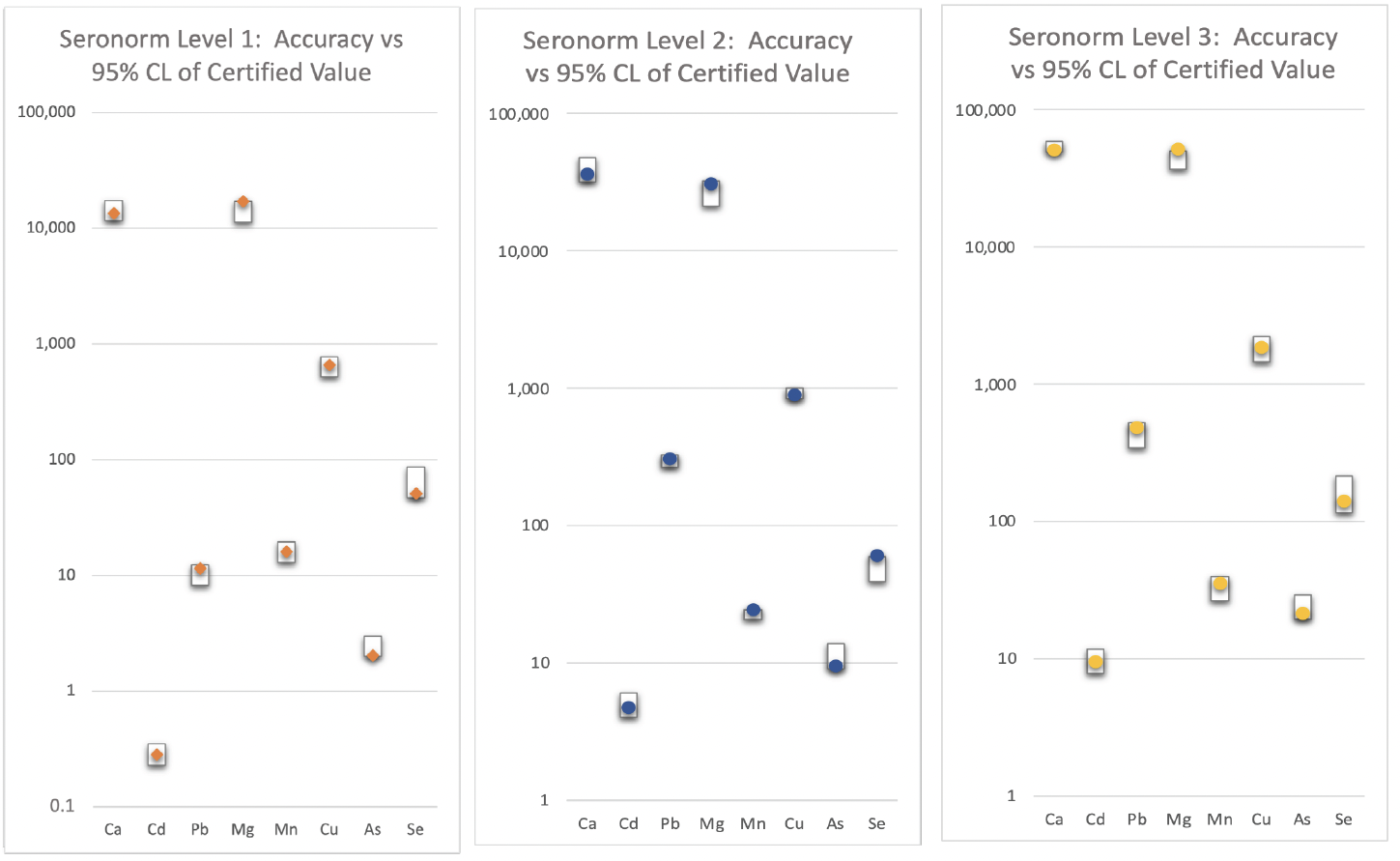
Figure 1: Accuracy of Certified Seronorm Material Analysis
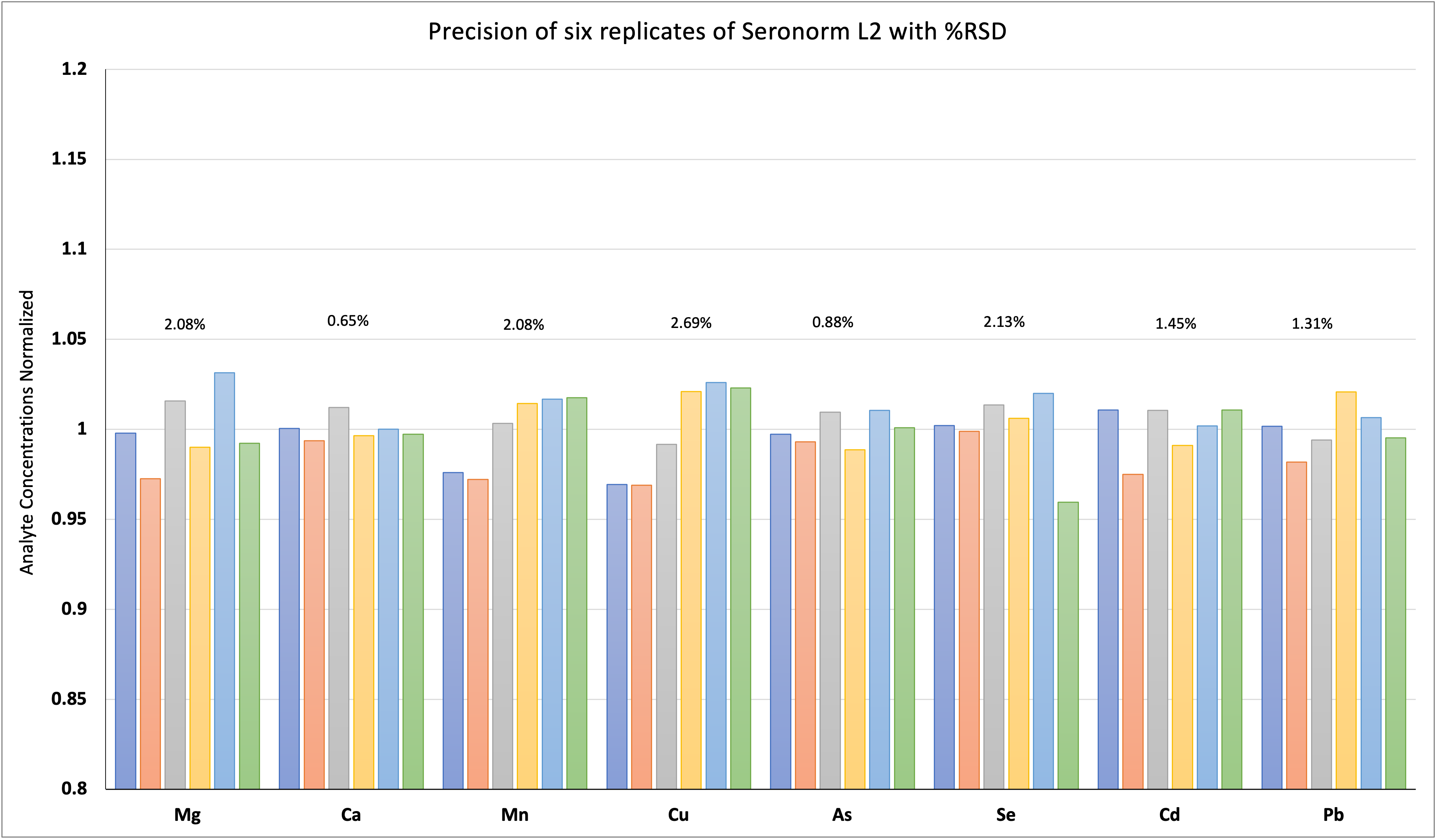
Figure 2: Precision of Seronorm Material Analysis
The method detection limit (MDL), and limit of quantitation (LOQ) were determined using the standard deviation (σ) of the signal from eight acid diluent blanks. The acid diluent blanks were prepared using the same technique and equipment as the samples and were included at the end of each run followed by a calibration standard. The MDL and LOQ are calculated as:
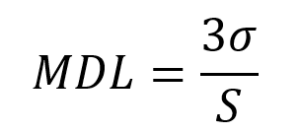
where S is the calibration slop, and:
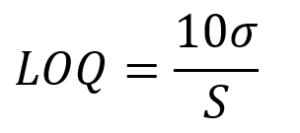
Since the calibration slope was the analyte relative to an internal standard, the calibration standard was used to determine the counts/second/ppb. These values are calculated, multiplied by 50 to account for the dilution factor, and expressed in μg/L (ppb). In the table, the MDL and LOQ are plotted relative to physiological normal values represented by Seronorm 2 (SRM2). For most analytes, the MDL and LOQ are miniscule by comparison, particularly for the major elements calcium and magnesium. However, even for more challenging analytes such as selenium, the MDL is an order of magnitude less than Seronorm 2 (SRM2) which, despite being level 2 (SRM2), has the lowest selenium content of these three SRMs.
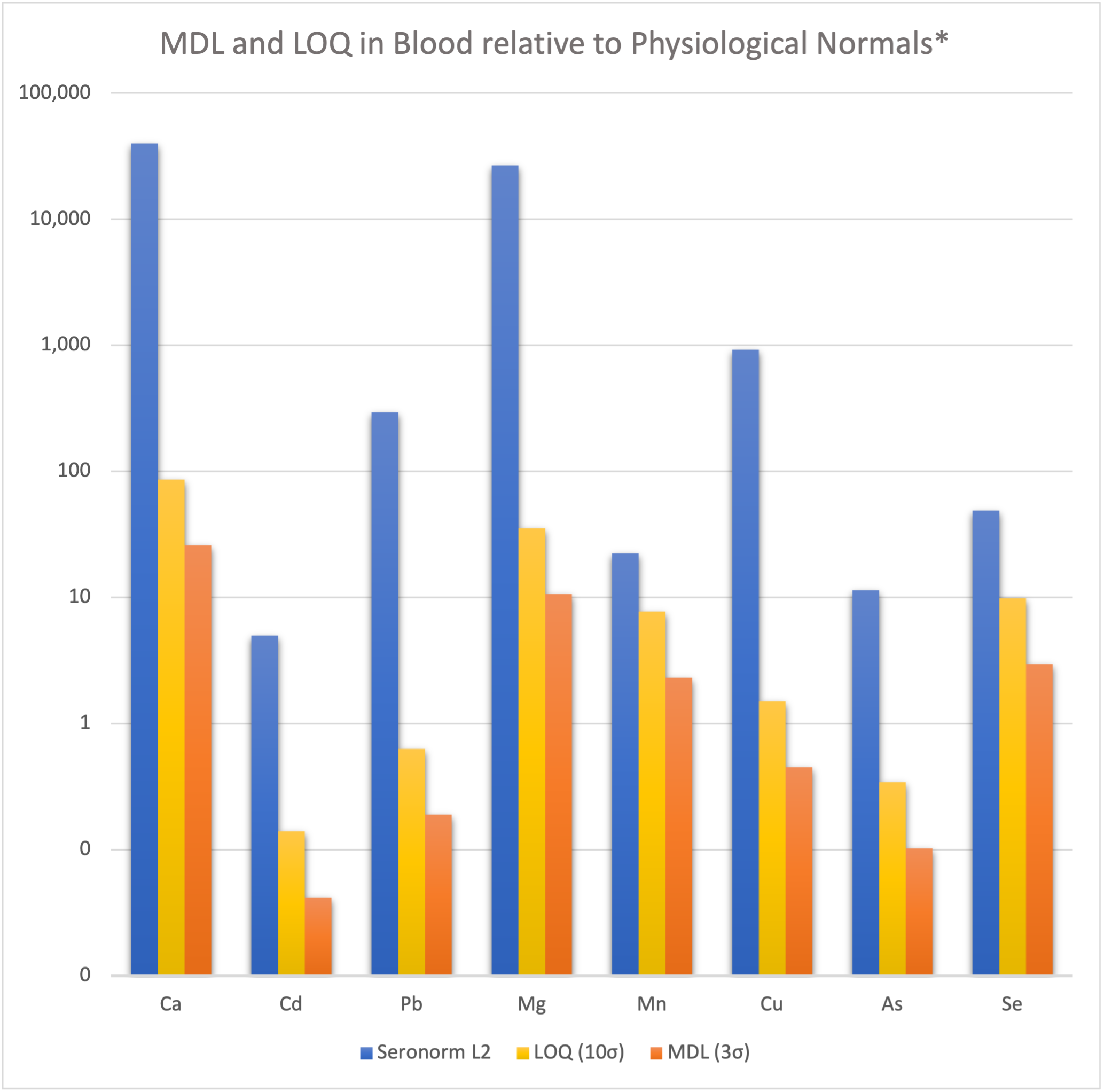
Figure 3: LOQ and MDL as compared to physiological normal values
*as represented by Seronorm L2, formulated to represent typical, or average human values.
Conclusion
In this application note, we report on the analysis of trace elements in blood using the Advion Interchim Scientific SOLATION® ICP-MS. Blood is a complex and challenging matrix. However, these data support the use of a straightforward “dilute and shoot” sample preparation method that gives accurate and precise concentrations for high level and trace level elements in blood. Excellent recoveries were observed for both spiked samples and CRMs. The combination of the quadrupole deflector and the collision cell minimizes drift and ensures accuracy and precision over time. The reported method benefits from the fast collision cell gas switching capabilities of the SOLATION® to analyze a wide range of elements in blood for rapid, accurate and reproducible results.
