VaDDRx 2022: Cardiovascular, Pulmonary, and Metabolic Disease
MIKIW, Medicinal Chemistry Conference
OHSU Chemical Biology and Physiology Conference
High-Throughput Purification of Five Over-the-Counter & Prescription Drug Compounds by Reverse-Phase Preparative LC-MS
Instrumentation:
puriFlash® 5.250
expression® CMS
Uptisphere® StrategyTM column US5C18HQ-150/300
Authors:
Advion Interchim Scientific, Montluçon, France Headquarters
Introduction
Purification is a critical step in drug development. From research, to scale-up to process, purification and confirmation are essential steps in bringing a drug to market. It is essential to have a high-throughput solution that offers sufficient quantity and reproducible quality of purified compounds. The separation of the active pharmaceutical ingredients (APIs) from their impurities can be easily achieved with a preparative chromatography system.
This application note features the purification of five active ingredients found in over-the-counter (OTC) drugs including caffeine, glafenine, ketoprofen, flavone, and fenofibrate (Figure 1), by a preparative purification workflow with confirmation using a compact mass spectrometer.

Figure 1: The five compounds of interest include caffeine, glafenine, ketoprofen, flavone, and fenofibrate. Chemical structures and pharmaceutical use cases are highlighted below.
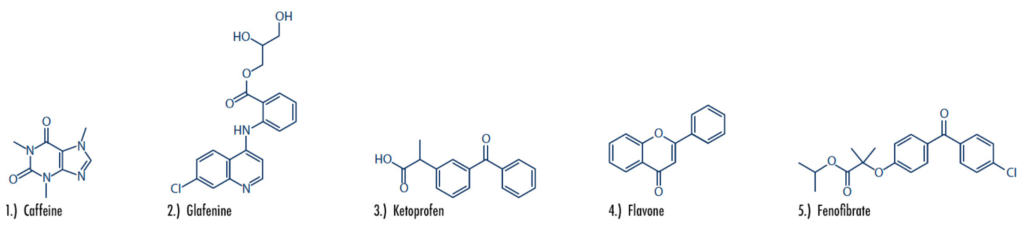
Caffeine: A natural chemical with stimulant effects, caffeine can be found purified in tablet form, or naturally occurring in coffee, tea, cocoa and more.
Glafenine: A nonsteroidal anti-inflammatory drug (NSAID), glafenine was removed from the market in 1991 due to a high risk of anaphylaxis.
Ketoprofen: A prescription-based nonsteroidal anti- inflammatory drug (NSAID), ketoprofen is used to treat inflammation, swelling, stiffness and joint pain. The drug was discontinued in 1995 due to increased risk of heart attack, stroke, irritation and other issues.
Flavone: A metabolite and nematicide that commonly exists in plants.
Fenofibrate: A prescription medication used to reduce and treat high cholesterol and triglyceride (fat-like substances) levels in the blood.
Experiment
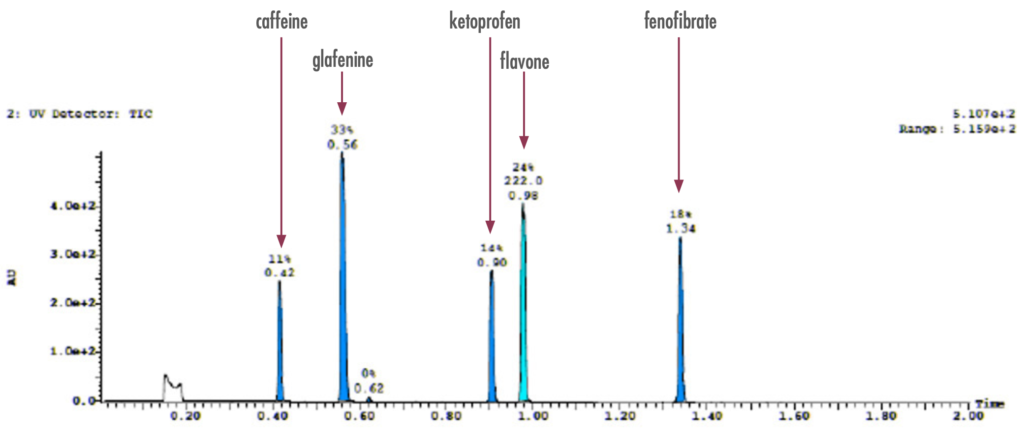
Exploratory LC Separation
Figure 2: To confirm the presence of the pre-identified compounds, an exploratory LC-UV run confirmed the presence of the drug compounds prior to purification.

Preparative LC Run
Following the positive ID of the five compounds of interest and their elution points, the drug mixture was then ready for a preparative LC-UV run on the puriFlash® 5.250 iELSD. The purification is aided by the iELSD pack, enabling the detection of chromophore-free compounds (Figure 3).

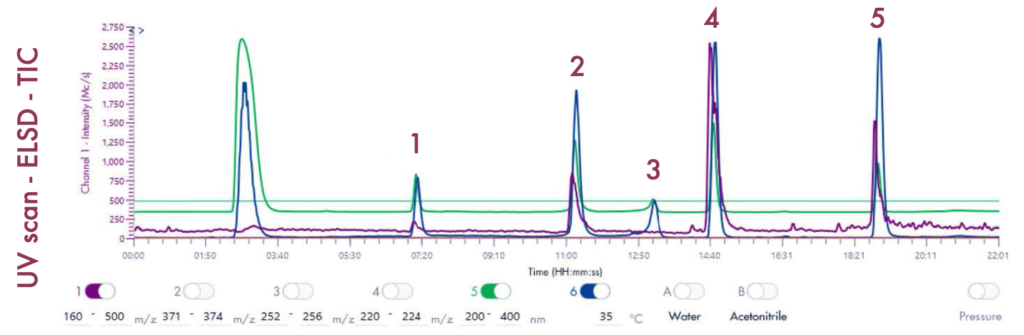
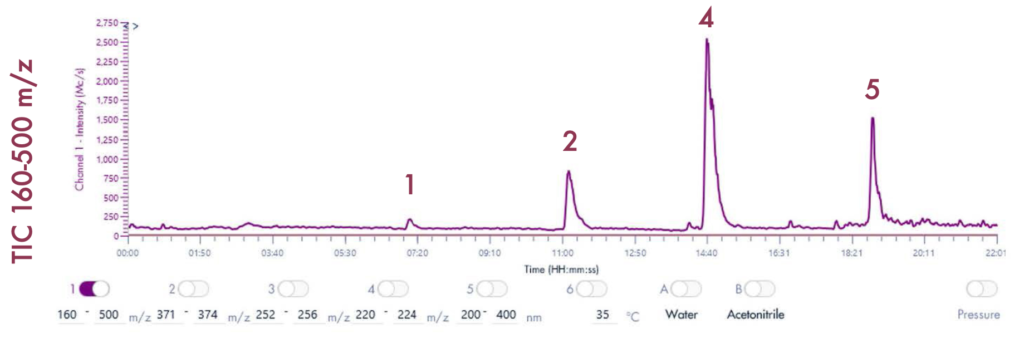
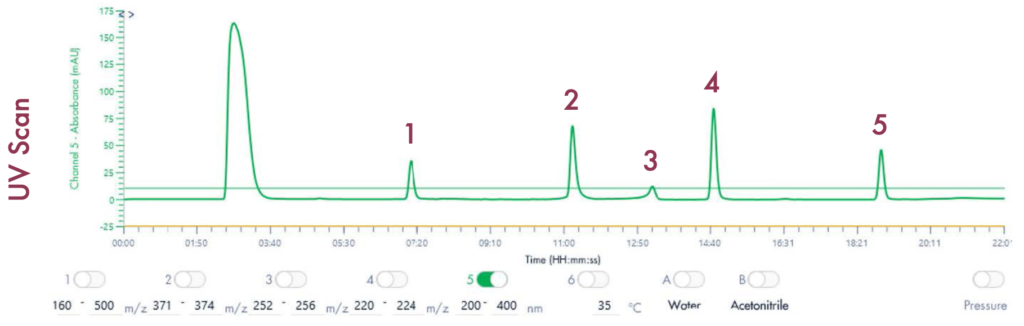
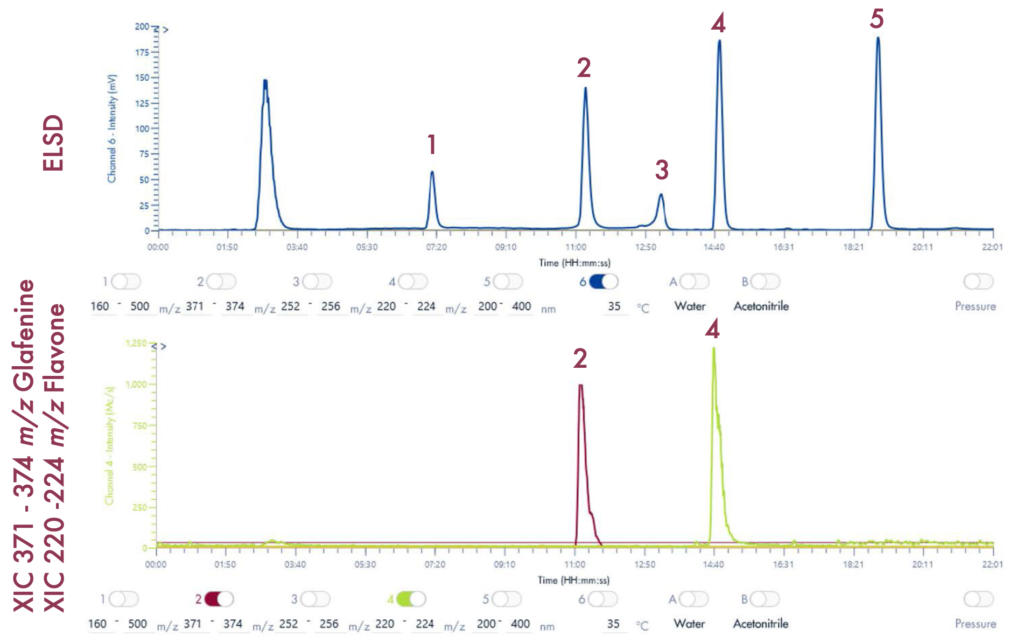
Results and Validation
Separation & Purification Results
The identity of the separated compounds was confirmed using the Advion Interchim Scientific expression® Compact Mass Spectrometer, quickly and accurately identifying the compounds of interest.
The purity of these compounds can be verified using analytical scale HPLC.

SOLATION®, A New ICP-MS for the Detection of Heavy Metals in Cannabis and Hemp
Introduction
Cannabis and hemp products are becoming much more available for medicinal and recreational use making routine testing for toxic heavy metals much more important. Advion Interchim Scientific introduces the SOLATION® ICP-MS for the analysis of heavy metals in cannabis plant and cannabis product samples. While there are no federal guidelines for heavy metals in cannabis, states where cannabis use and production are legal have adopted exposure limits and QC criteria for Arsenic, Cadmium, Mercury, and Lead based on USP<233>. Here, we report the results of our sample analysis using these guidelines.
Methods
Cannabis flower was purchased locally and finely ground for analysis. Samples are prepared using a microwave digestion system (CEM Mars 6, Matthews, NC). Method validation for USP<233> is based on accuracy, using spike recoveries, repeatability based on the %RSD of six independently digested replicate, and ruggedness, where those 6 replicates are run a second time by another analyst, another instrument, or on another day. The spike levels are based on the “action level” defined by the California maximum permitted daily exposure (PDE) limits as a guide: Lead 0.5 µg/g, Arsenic and Cadmium 0.2 µg/g, and Mercury 0.1 µg/g are used to define the 100% spike level. Samples are also spiked at 50% and 150% of the action level.
Preliminary Data
For digestion, 0.5 g (+/- 0.002g) of sample is treated with 9mL conc. HNO3 and 1mL conc. HCl in a microwave vessel and allowed to react for 15 minutes prior to being capped. The vessels are loaded onto the carousel in the microwave and the “one touch” cannabis method, supplied by CEM, is used. Samples are brought to 200°C in 30 minutes, held there for 10 minutes, and allowed to cool. The result is a clear, particle free solution. The SOLATION® ICP-MS was used to analyze the samples for As, Cd, Hg, and Pb after digestion and dilution. The results show that the SOLATION® ICP-MS was able to produce accurate values as measured by the spike recoveries which were well within the 70-150% range. The results from the 6 independent digests were within the defined limit of 20% RSD. Repeat analysis of the 6 digests on a separate day showed good agreement with the initial results and were within the 25% RSD spec. defined by USP<233>. Overall results show that the SOLATION® ICP-MS is an effective instrument for the analysis of cannabis and hemp samples.

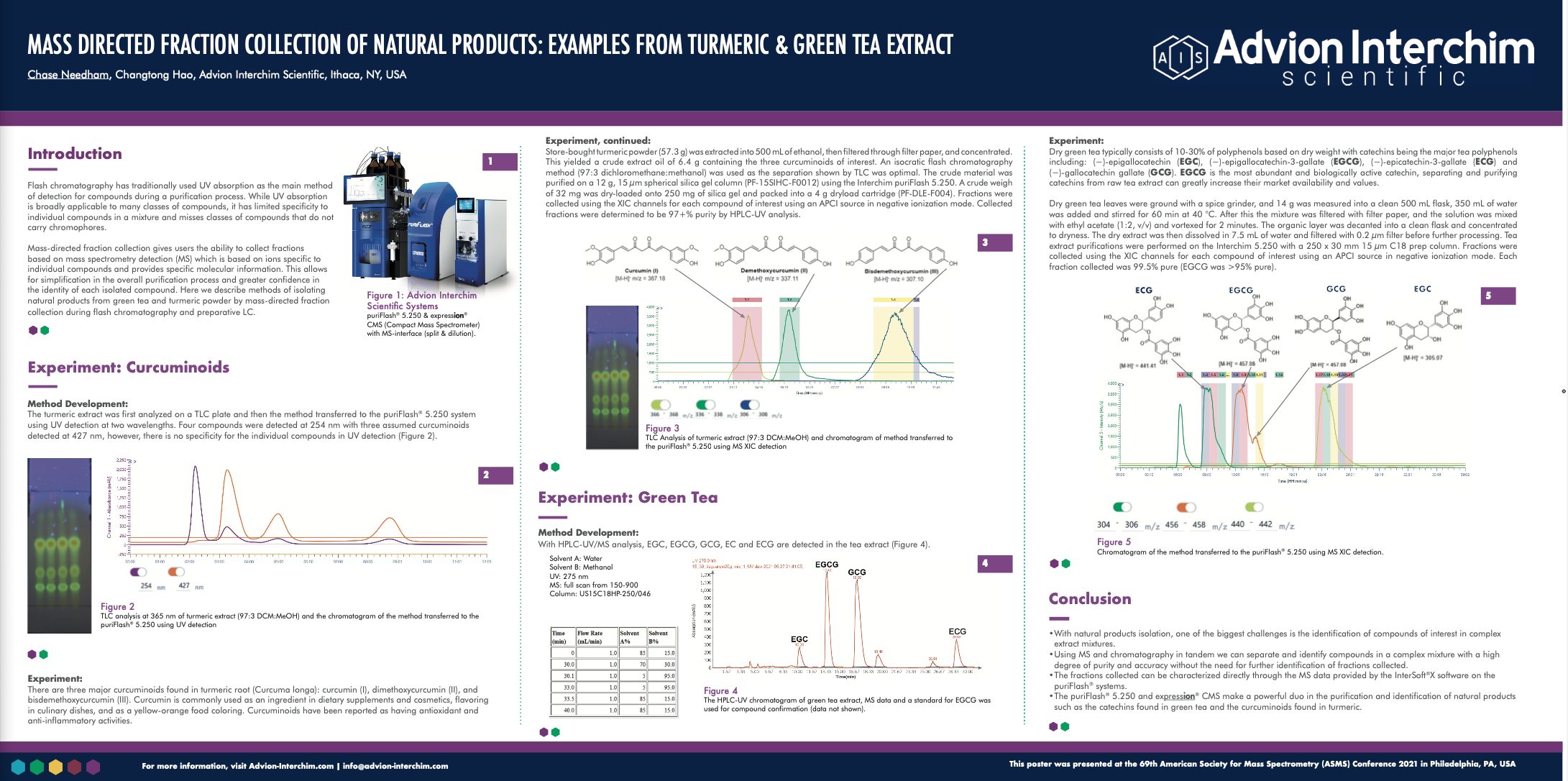
Mass Directed Fraction Collection of Natural Products: Examples from Turmeric & Green Tea Extract
Introduction
Natural products have been a source of inspiration for pre-clinical drug discovery both by exploring traditional medicines and to discover new spaces in pharmacology. Isolation and characterization of natural products remains a major barrier in drug discovery. Isolation is generally done on an analytical scale and then compounds are characterized fully before any scaleup purifications are attempted. The ability to purify compounds in complex natural product mixtures by highly specific MS data allows for the simplification of the purification and characterization steps of the process. Here, flash and prep chromatography are coupled with MS detection to purify natural products in green tea and turmeric extracts.
Methods
Isolation of the major catechins in green tea and the major curcuminoids in turmeric was completed via extractions. Green tea leaves were extracted, and the crude material was analyzed using UPLC-MS and analytical standards to identify the catechins of interest and develop a suitable prep-LC method for isolation. Turmeric powder was extracted and then analyzed by TLC-MS using Advion’s Plate Express™ to identify the compounds of interest and develop a suitable flash chromatography method for isolation. Both extracts were purified using mass-directed fraction collection using Interchim’s puriFlash 5.250 flash/prep chromatography system connected to Advion’s expression® single quadrupole mass spectrometer. Target compounds were detected using XIC MS channels. Purity of the isolated compounds was then determined using HPLC-MS.
Preliminary Data
We were able to successfully isolate the 3 major curcuminoids (curcumin, demethoxycurcumin, and bisdemethoxycurcumin) in turmeric and the 5 major catechins in green tea ((-)-epigallocatechin (EGC), (-)-epicatechin (EC), (-)-epigallocatechin-3-gallate (EGCG), (-)-epicatechin-3-gallate (ECG), and (-)-gallocatechin gallate (GCG) with high purity (≥95%).
An isocratic flash chromatography method (97:3 DCM:MeOH) was developed using TLC (97:3 DCM:MeOH) to purify the curcuminoids. The TLC plate was analyzed by APCI– MS using the Plate Express which extracts spots directly from the plate with no need for sample preparation. Fractions were collected using extracted ion chromatogram (XIC) channels with APCI– MS. Fractions were then characterized by ASAP– MS and purity for each fraction was determined using HPLC-MS.
A preparative LC method was developed for the catechins in green tea using HPLC-MS and reference standards to identify each compound of interest. Using a water methanol gradient and collecting fractions using XIC channels set for the compounds of interest. Fractions were characterized and their purity determined by HPLC-MS. EGC, EGCG, GCG, and ECG fractions were determined to have purities of 100 %, 99.8 %, 98.8, and 100 % respectively.
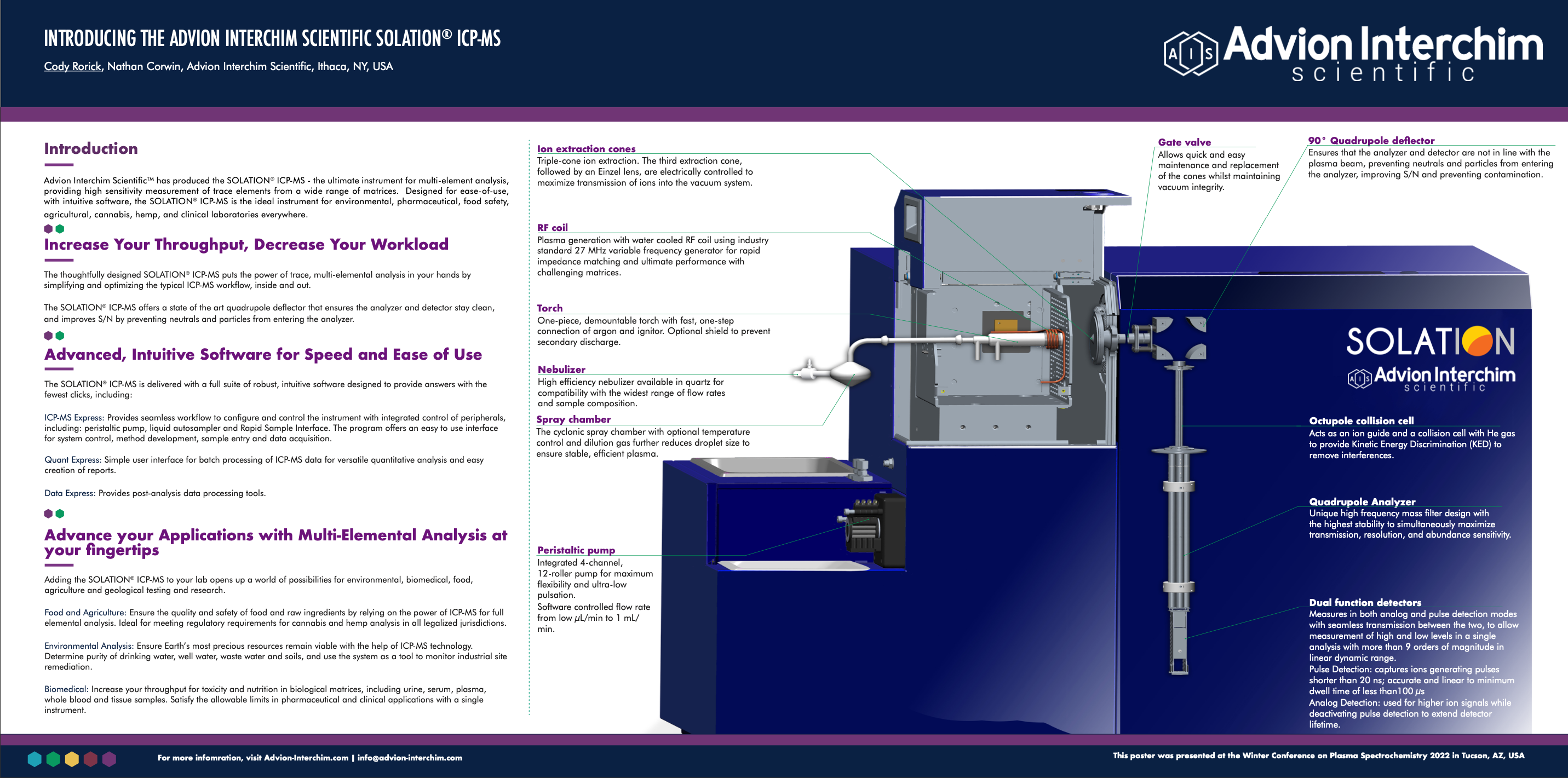
Introducing the Advion Interchim Scientific SOLATION® ICP-MS
Introduction
The SOLATION® Inductively Coupled Plasma Mass Spectrometer (ICP-MS) puts the power of trace, multi-elemental analysis in your hands by simplifying and optimizing the typical ICP-MS workflow, inside and out. The system offers high performance, multi-elemental analysis, ideal for environmental, clinical, biomedical, food, agriculture, and geological applications.
The SOLATION® offers a state-of-the-art quadrupole deflector that ensures the analyzer and detector stay clean and improves S/N by preventing neutrals and particles from entering the analyzer. The system is also designed for lower argon consumption.
Key System Infrastructure Includes:
- Ion extraction cones: Triple-cone ion extraction, followed by an Einzel lens, which are electrically controlled to maximize transmission of ions into the vacuum system.
- RF coil: Plasma generation with water cooled RF coil using industry standard 27 MHz variable frequency generator for rapid impedance matching and ultimate performance with challenging matrices.
- Torch: One-piece, demountable torch with fast, one-step connection of argon and ignitor. Optional shield to prevent secondary discharge.
- Nebulizer: High efficiency concentric nebulizer available in glass or quartz for compatibility with the widest range of flow rates and sample composition.
- Spray chamber: The cyclonic spray chamber with optional temperature control further reduces droplet size and solvent load to ensure stable, efficient plasma.
- Peristaltic pump: Integrated 4-channel, 12-roller pump for maximum flexibility and ultra-low pulsation. Software controlled flow rate from 1 μL/min to >1 mL/min.
- Gate valve: Allows quick and easy maintenance and replacement of the cones whilst maintaining vacuum integrity.
- 90° Quadrupole deflector: Ensures that the analyzer and detector are not in line with the plasma beam, preventing neutrals and particles from entering the analyzer, improving S/N and preventing contamination.
- Octupole collision cell: Acts as an ion guide and a collision cell with He gas to provide Kinetic Energy Discrimination (KED) to remove interferences.
- Quadrupole Analyzer: High frequency mass filter design with the highest stability to simultaneously maximize transmission, resolution, and abundance sensitivity.
- Dual function detectors: Measures in both analog and pulse detection modes with seamless transmission between the two, to allow measurement of high and low levels in a single analysis with more than 9 orders of magnitude of linear dynamic range.
- Pulse Detection: captures ions generating pulses shorter than 20 ns; accurate and linear to minimum dwell time of less than 100 μs
- Analog Detection: used for higher ion signals while pulse detection is deactivated to extend detector lifetime.
- Mass Dependent software control: Software designed to optimize specific mass ranges independently to allow for mass specific tune optimization.