
Mass Spec: expression® CMS
Sampling: Plate Express™
In the spirit of the Holiday season and to ensure that mistletoe kisses are enjoyed and are ‘non-toxic’, we employed the Advion Interchim Scientific expression® Compact Mass Spectrometer (CMS) and the Plate Express™ TLC Plate Reader to analyze a commercial Tincture of Mistletoe ethanolic extract to determine whether tyramine is present in the extract of mistletoe.
INTRODUCTION
A sprig of mistletoe symbolizes a tradition of romance (Figure 1), and has a legacy of folklore purporting that extracts of mistletoe can cure cancer along with a long list of other reported health benefits. However, mistletoe is also considered lethal. Reputed to be the “kiss of death”, mistletoe is said by some to be so poisonous that humans can be killed if they ingest the leaves or berries.
Figure 1: The tradition of mistletoe.

The reported toxicity made us wonder, why or how can vendors sell mistletoe extracts for purposeful human consumption? One species of mistletoe, Viscum, reportedly contains the poisonous alkaloid, tyramine, which can cause blurred vision, nausea, abdominal pain, diarrhea, blood pressure changes, and even death. A search of peer-reviewed scientific literature reveals a dearth of credible analytical support for the presence of tyramine in mistletoe.
In the spirit of the Holiday season and to ensure that mistletoe kisses are enjoyed and are ‘non-toxic’, we employed the Advion Interchim Scientific TLC/CMS system (Figure 2) to analyze a commercial tincture of Mistletoe ethanolic extract to determine whether tyramine is present in the extract of mistletoe.
Figure 2: Experimental setup of the Advion Interchim Scientific expression® CMS with the Plate Express™ TLC Plate Reader.

Figure 3: Experimental herbs used.

EXPERIMENTAL
A tincture of mistletoe was purchased from Indigo Herbs. A small aliquot of this tincture sample was derivatized with dansyl chloride at 50 ºC for 30 min according to well-known procedures[1]. Similarly, an authentic sample of tyramine was derivatized in the same manner to form its dansyl derivative.
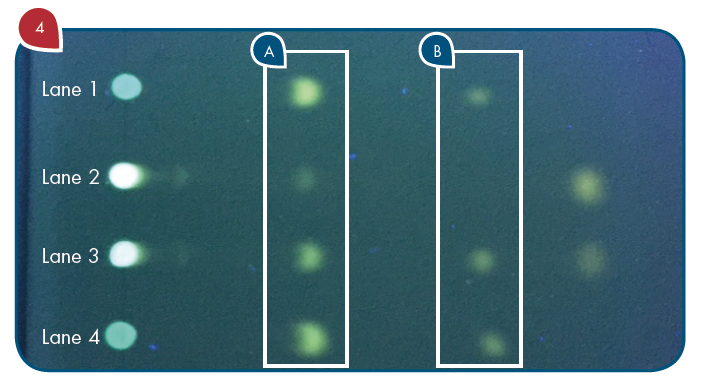
A small aliquot (10 mL) of the standard tyramine dansyl derivative was applied to the outside lanes (Lanes 1 and 4) of a Merck Silica gel G TLC plate. An aliquot of the derivatized tincture of mistletoe was applied to Lane 2 and a derivatized tincture of mistletoe spiked with tyramine dansyl derivative was applied to Lane 3 (Figure 4).
Figure 4: TLC plate after development and visualization under long wavelength UV light. Lanes 1 and 4: Dansyl derivative of standard tyramine. Lane 2: Dansyl derivative reaction mixture of mistletoe tincture sample. Lane 3: Tincture extract dansyl derivative with standard tyramine dansyl derivative spiked into it. (A) Rf=0.3 for tyramine dansyl derivative. (B) Rf=0.6 for dansyl chloride.
The air-dried TLC plate was developed in an equilibrated solvent tank containing chloroform/ethyl (8/2, v/v) acetate. The developed TLC plate was then viewed under long wavelength UV light to reveal the separated components (Figure 3). The TLC plate was positioned onto the Plate Express™ TLC Plate Reader whereupon each TLC ‘spot’ could be individually analyzed by TLC/CMS.
With reference to Figure 4, the TLC/CMS analysis readily showed that the Rf 0.3 spots in the two outside lanes (Lanes 1 and 4) produced a mass spectrum with an abundant m/z 371 consistent with the expected protonated molecule of the tyramine dansyl derivative (Figure 5A). The TLC/CMS mass spectra obtained from the spots with an Rf=0.6 observed in Lanes 1 and 4 were consistent with unreacted dansyl chloride with a protonated molecule at m/z 270 (data not shown). TLC/CMS analysis of the spot in lane 2 at Rf=0.3 showed no evidence for the presence of tyramine dansyl derivative (Figure 5B).
Figure 5: (A) TLC/CMS mass spectrum of standard tyramine dansyl derivative observed at Rf=0.3 in Figure 4 Lane 1. (B) LC/CMS mass spectrum of derivatized tincture of mistletoe observed at Rf=0.3 in Figure 4 Lane 2.
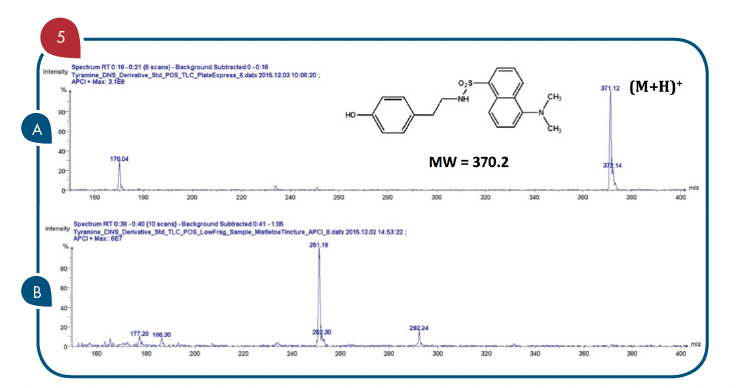
In the absence of TLC/CMS analysis, it would be logical to conclude the spot at Rf=0.3 in lane 2 was due to the presence of tyramine in the mistletoe tincture sample. The Rf=0.3 spot observed for the fortified tincture extract in Lane 3 of Figure 4 readily showed the same mass spectrum for tyramine dansyl derivative that is shown in Figure 5A. The same negative results for tyramine were obtained from the alcohol extract of the mistletoe leaf product.
CONCLUSIONS
The results from this brief study suggest either that the level of tyramine in the tincture sample is very low and below our detection limits or that tyramine is not present in the sample. It is common for synthetic and forensic chemists to employ TLC techniques as a quick, easy screen of a sample to determine the presence of an expected chemical. Comparison with a known sample, which shows the same Rf value, will often provide some confidence for reporting the presence of the expected compound. However, as this example suggests a similar Rf value does not guarantee confirmation of the spot identity when it has the same Rf value. As shown here, access to the direct analysis of the spot with the Advion Interchim Scientific expression® CMS can either corroborate the expected identification or, as in this case, suggest that the spot with the same Rf value is NOT the expected compound. These results may explain why the commercial mistletoe tincture samples are not harmful for medicinal purposes. So, what should you do? Mistletoe is not deadly. But it can be hazardous, so don’t eat it. Just ‘steal a kiss under it’!
REFERENCES AND ACKNOWLEDGEMENT
[1]Mullins, Donald E. and Eaton, John L. Quantitative high-performance thin-layer chromatography of dansyl derivatives of biogenic amines, Anal. Biochem., 1988, 172, (484-487).
Thank you to Chief Elf, Nigel Sousou, Ph.D., for leading the sample analysis process.








 Kahen will immediately become CEO of the Advion group. David Patteson, the prior Chairman and CEO of the group will remain as group Chairman. Dr. Kahen will be closely involved in the proposed combination of Advion and Interchim, presently underway and as previously announced, and would assume a resulting executive leadership position.
Kahen will immediately become CEO of the Advion group. David Patteson, the prior Chairman and CEO of the group will remain as group Chairman. Dr. Kahen will be closely involved in the proposed combination of Advion and Interchim, presently underway and as previously announced, and would assume a resulting executive leadership position.