Eastern Analytical Symposium (EAS) 2021
American Society of Mass Spectrometry (ASMS) Annual Conference 2021
ACS Fall National Meeting 2021
SupplySide West 2021
North American Chemical Residue Workshop (NACRW) 2021
ACS Middle Atlantic Regional Meeting (MARM) 2021
ACS Great Lakes Regional Meeting (GLRM) 2021
puriFlash-MS Targeted Isolation of Natural Products Under Normal Phase Conditions
Introduction

The improvement of analytical techniques and methodological tools plays an important role in the characterization and isolation of bioactive secondary metabolites in natural product research. Reverse-phase liquid chromatography-mass spectrometry (RP-LC-MS) is widely used for metabolite profiling of complex natural extracts at the analytical level and is increasingly being used for targeted MS isolation of biomarkers. Normal-phase chromatography (NP-LC) is well suited for the purification of polar secondary metabolites offering also some advantages compared to RP like low operating pressures and cheapest stationary phases.
NP-LC however is typically not well-suited for MS coupling. The potential of NP-LC-APCI-MS for metabolite purification at the preparative scale using generic separation methods has been investigated on an Advion X Interchim PuriFlash® – CMS system in view of its application for targeted MS isolation of lipophilic secondary metabolite. A mixture of three representative apolar natural products was used to optimize separation, splitting and MS ionization in conditions mimicking real isolation cases. Finally, successful isolation of the apolar constituents of the dichloromethane roots extract of Angelica archangelica was performed.
Rapid Isolation of a Representative Apolar Natural Product Mixture by Normal-Phase Flash-CMS
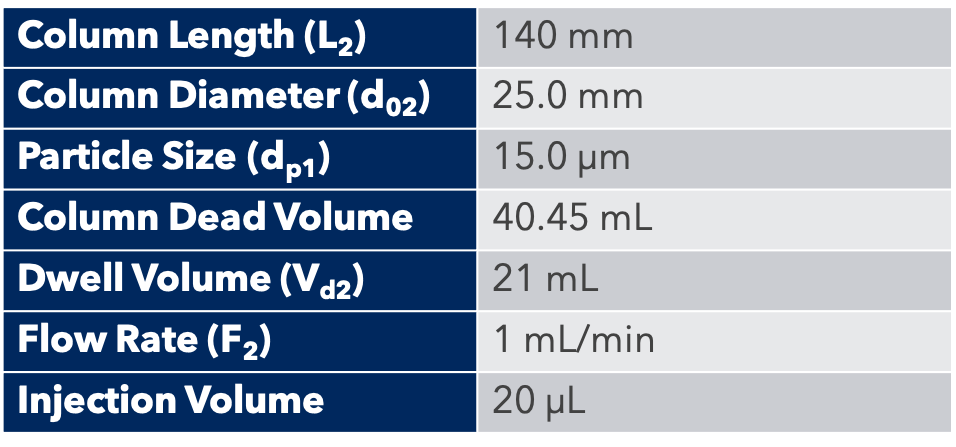
Purification of a Natural Products Mixture on 12 g and 25 g Normal Phase Flash Columns
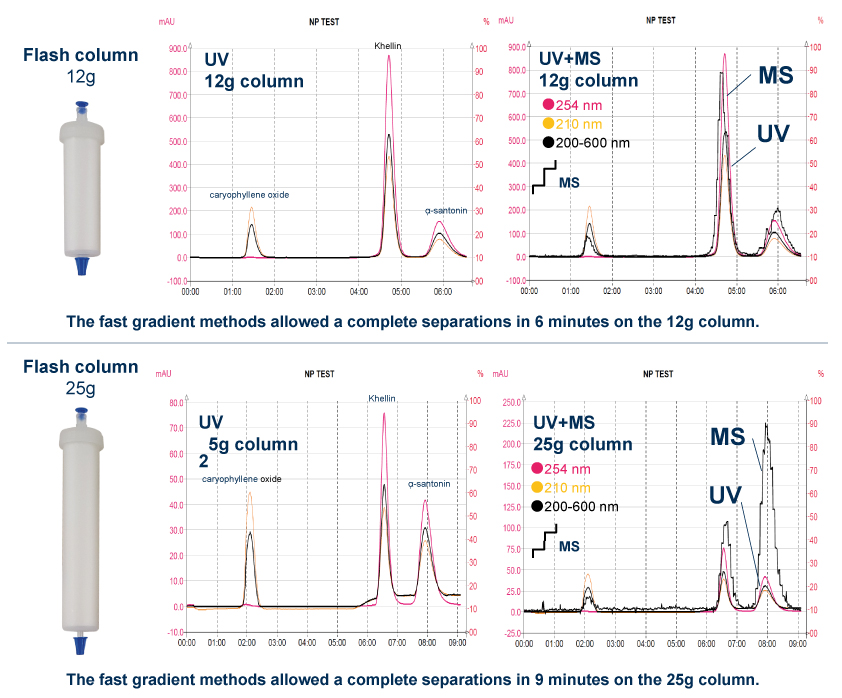
Three commercially available standards (caryophyllene oxide, khellin, and alpha-santonin) were used to evaluate the applicability of the puriFlash-CMS system as a tool for rapid purification of lipophilic compounds from crude plant extracts.
The four chromatograms show the mixture profile at the preparative scale with rapid gradients on two column sizes with a good overlap of the UV and MS signals.
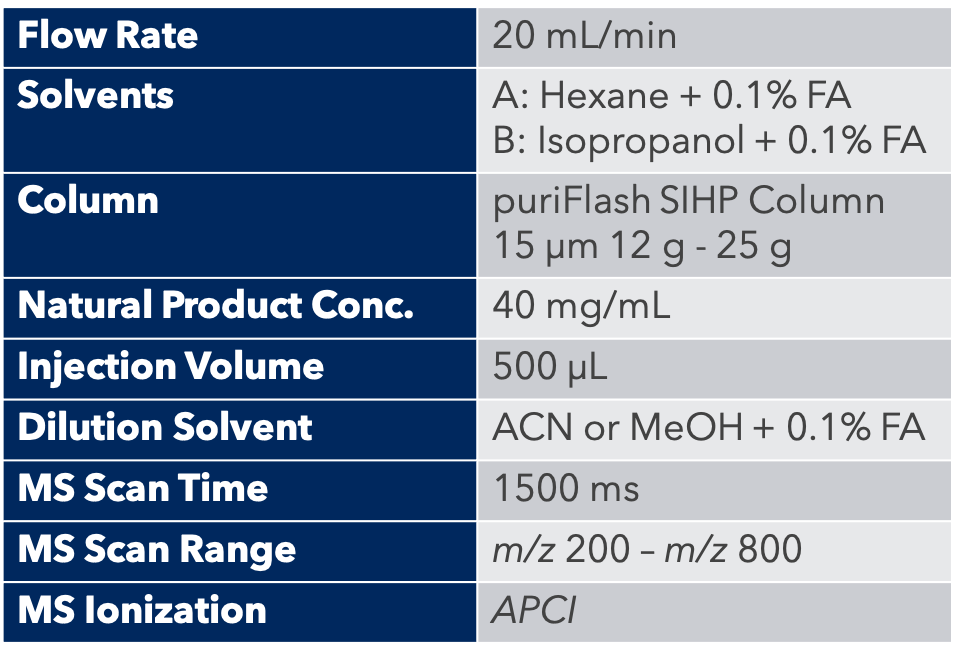
All parameters were carefully optimized for both separation and detection. Special care was taken to find ionization and splitting conditions that would provide good detection and
Flash-MS Guided Purification of a Given Compound
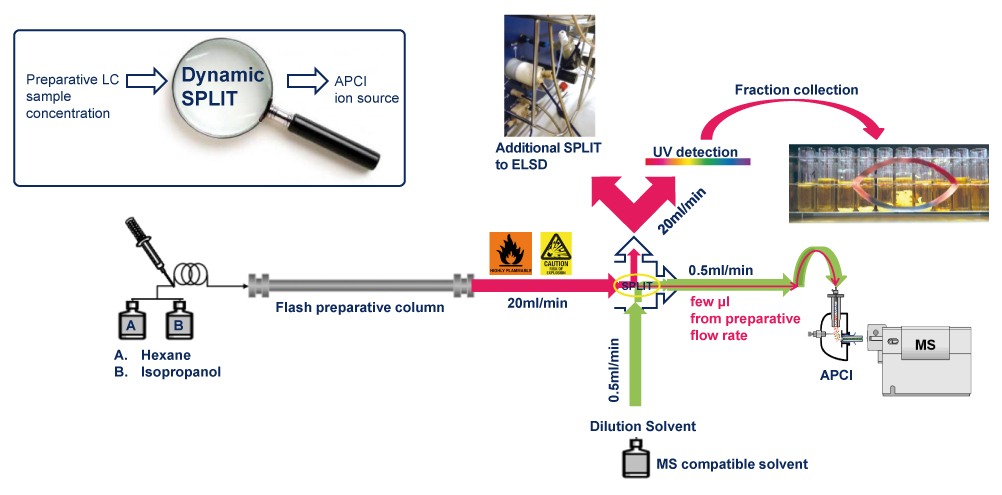
Scheme of the Post Column Makeup Pump Dilution

puriFlash-CMS System
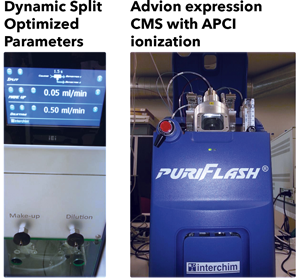
Solvents from the normal phase are highly flammable for the APCI source and should be avoided because of the heating process.
Optimized post-column dilution was mandatory in order to have an efficient and safe ionization. The solvent mixture when it reached the MS detector was at > 99% either ACN or MeOH.
APCI-MS detection with optimized splitting conditions and post-column elution of appropriate solvent was found robust and well suited for this type of purification.
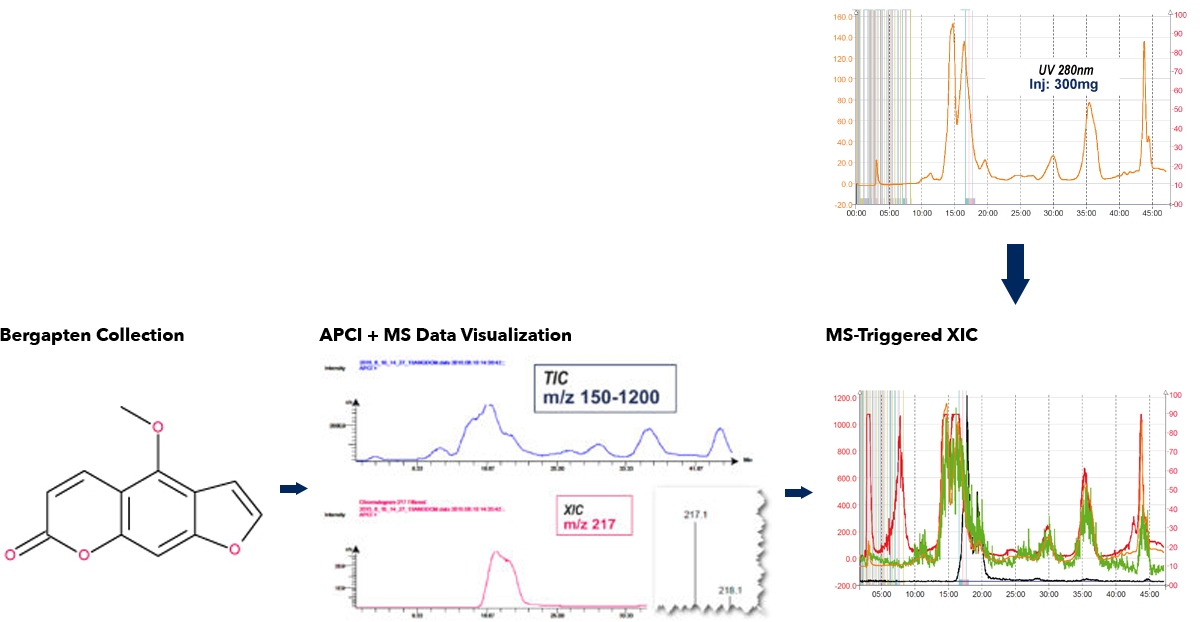
Normal-Phase puriFlash-CMS Purification of Angelica archangelica Roots Extract

Analytical HPLC-UV
 |
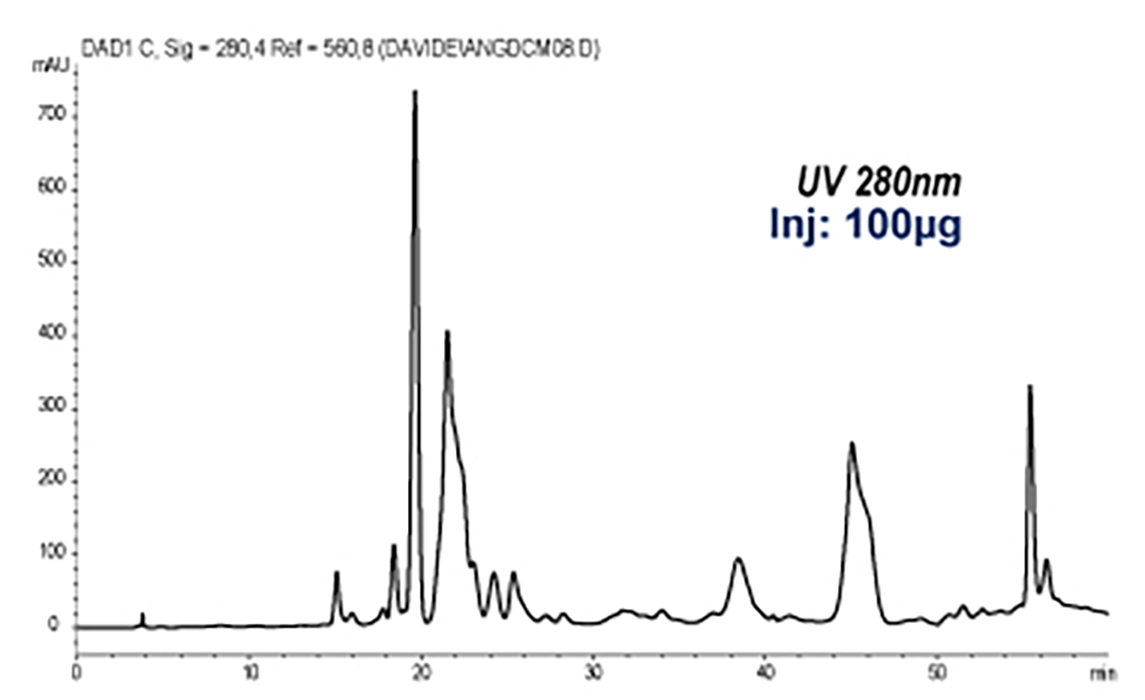 |
Analytical Scale:
 |
 |
Preparative Scale:
 |
 |

Flash Preparative UV-ELSD-MS
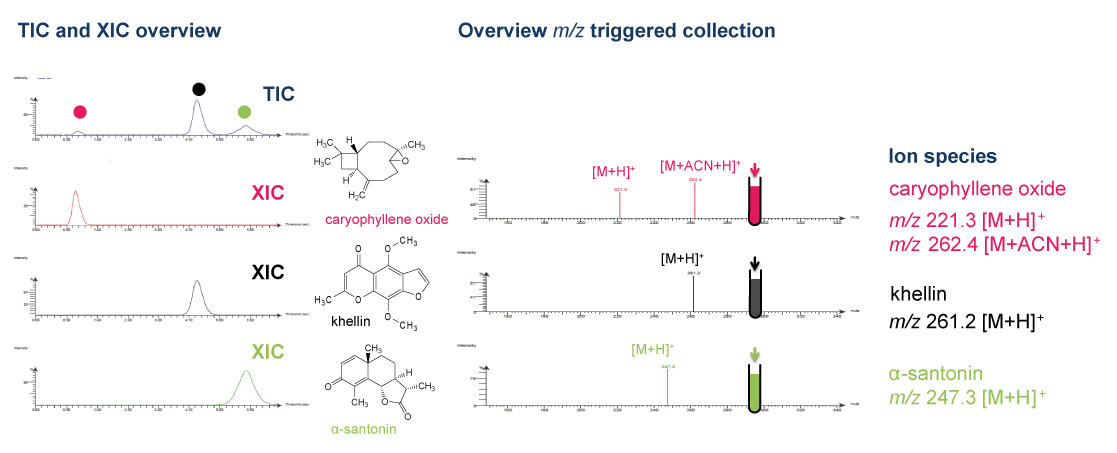
The roots of Angelica archangelica are rich in coumarin derivatives. MS-ELSD detection in addition to UV detection enabled the monitoring of secondary metabolites with no or weak chromophores and the selectivity of the MS was of great help for a precise collection of partially coeluting compounds.
Conclusion
Normal-phase flash purification represents an efficient strategy for a rational isolation of specific lipophilic biomarkers or bio-active compounds based on metabolite profiling results. MS-triggered fractionation and ELSD monitoring in addition to standard UV detection is a powerful tool for precise collection and to estimate the amount of separated compounds. MS is particularly useful for the specific collection of any given m/z in case of coelution that often occurs in crude extracts using high loading and low peak capacity chromatographic methodologies.
This fast and rational approach can be widely used for single step purifications and isolation of synthetic and natural mixtures. It is also compatible for the detection of apolar compounds that lack chromophores which is very common in natural product research. Separation performed at the preparative scale allows to purify tens to hundreds mg of compounds for further structural identification and assessment of their bioactivities.
References
Davide Righi1, Antonio Azzollini1, Emerson Ferreira Queiroz1, Jean-Luc Wolfender1
School of pharmaceutical sciences, University of Geneva, University of Lausanne, 30 Quai Ernest-Ansermet, 1211 Geneva 4, Switzerland
[1] Davy Guillarme, Dao T.T. Nguyen, Serge Rudaz, Jean-Luc Veuthey, Eur. J. Pharma. Biopharma. 2008, 68, 430
Peptide Purification: Flash-MS Coupling using the puriFlash 5.250P and expression CMS

In order to adapt, Interchim set up a laboratory to be able to perform online demonstrations. These demonstrations can be articulated around purifications of “standard” products in order to demonstrate the potential of our instruments. This example demonstrates the capabilities of a flash-ms system featuring the Interchim puriFlash 5.250 and Advion expression Compact Mass Spectrometer (CMS) for the purification of a peptide.
Installation
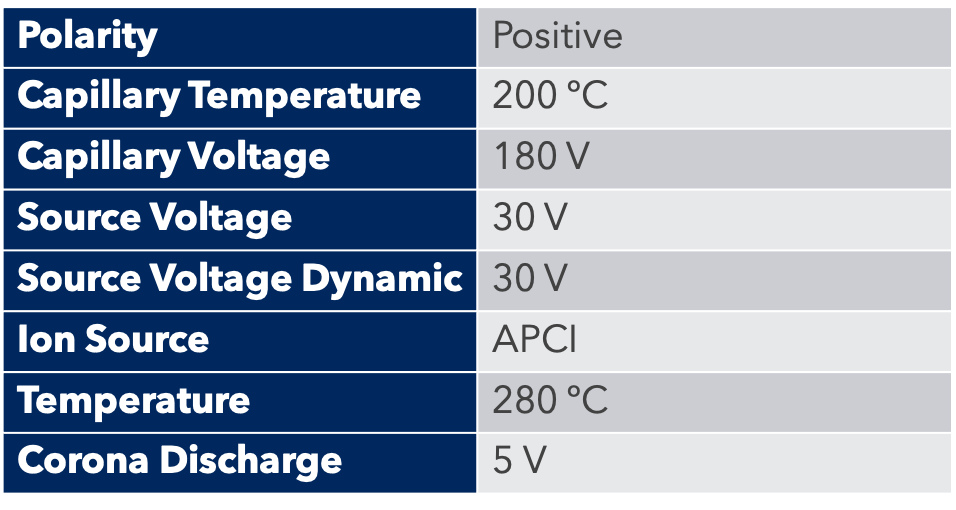
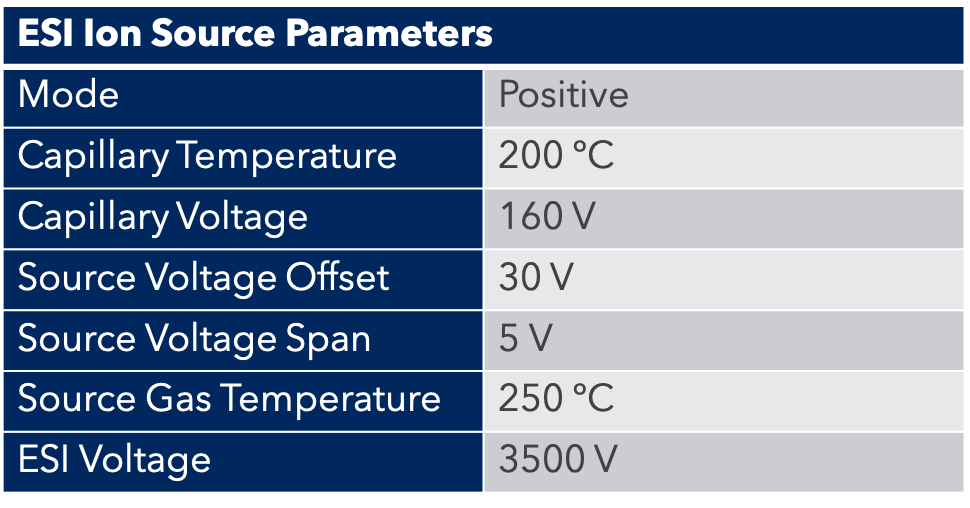
For the purification of a peptide resulting from chemical synthesis, we used the puriFlash 5.250P. To monitor this purification, we used a UV detector and Advion expression CMS.
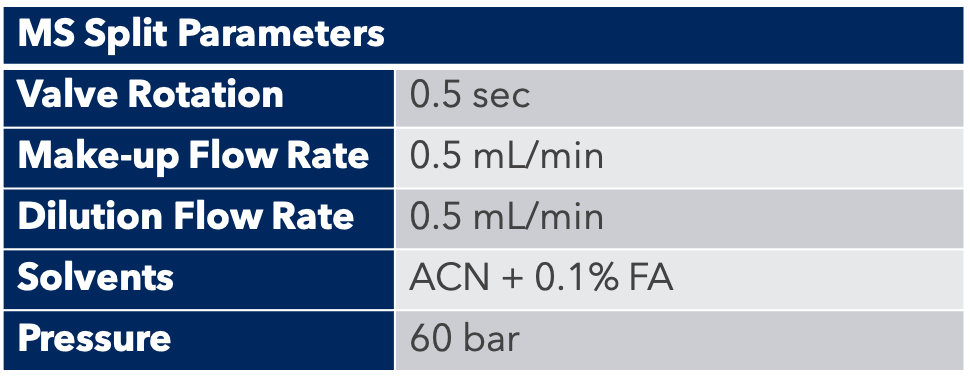
The detection of peptides by mass spectrometry is done with an ESI source, which is ideal for the detection of large molecules such as peptides. A small amount of product is “diverted” by the MS interface for detection.
Flow Injection Analysis (FIA)
Direct Injection of Crude Sample on the expression CMS.
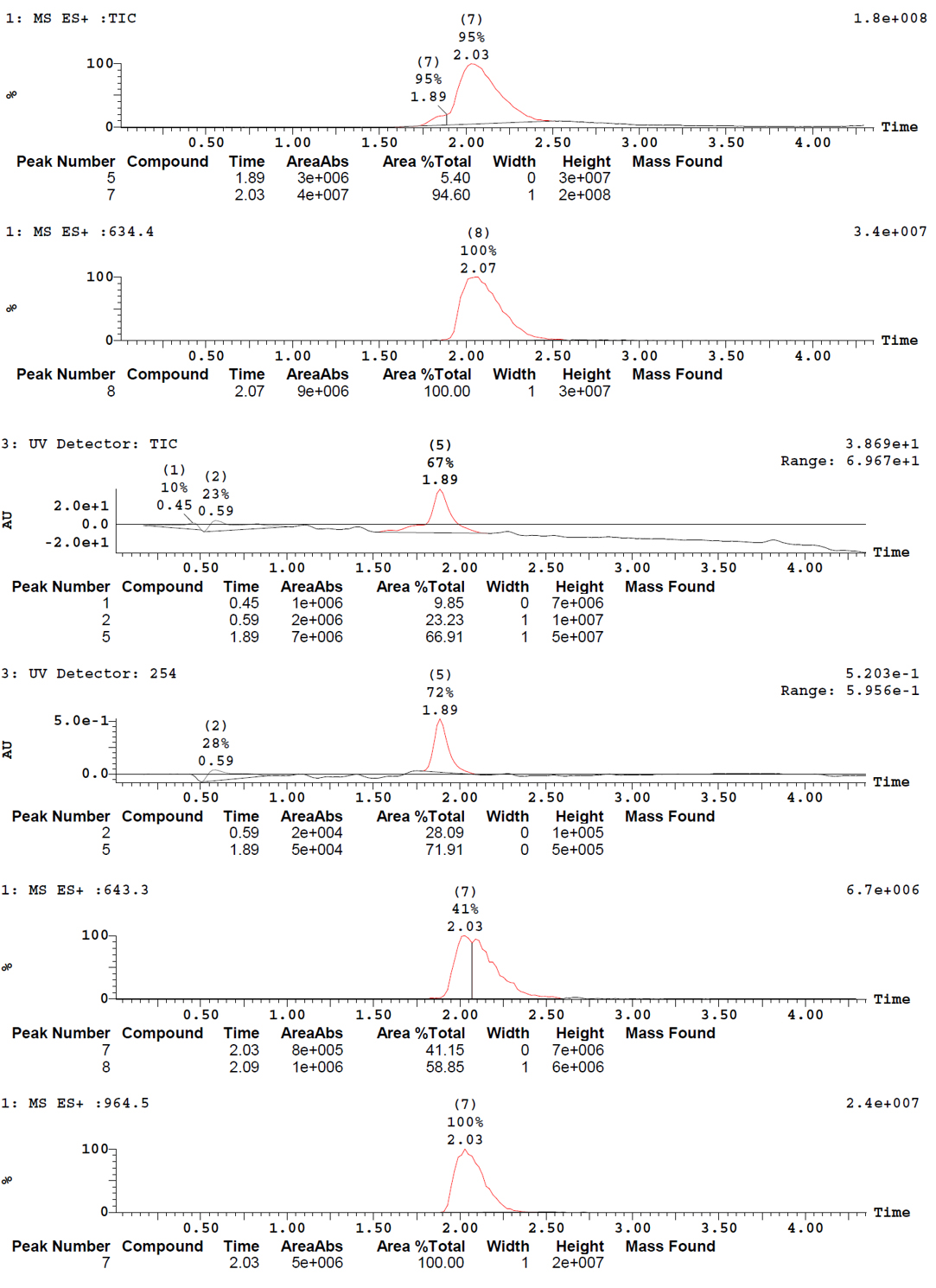
Here is the resulting mass spectra:
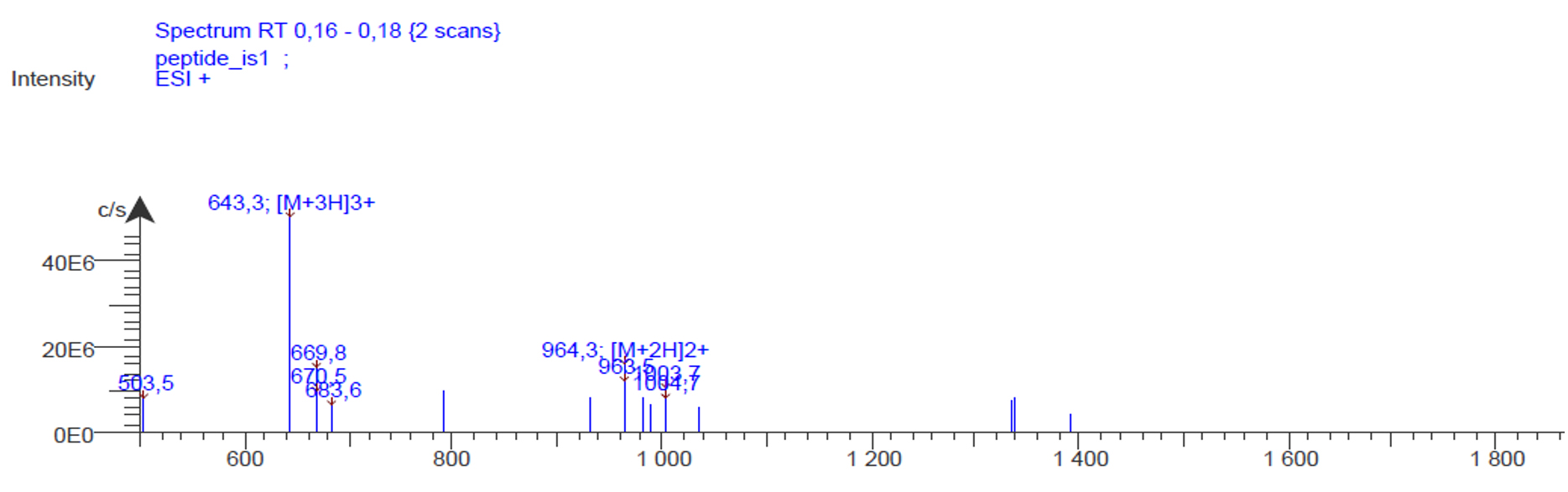
We observe a peak at m/z 643, which corresponds to the molecule of interested charged 3 times (M+3H)3+, as well as a peak at m/z 964.3, which corresponds to the molecule of interest charged 2 times (M+2H)2+.
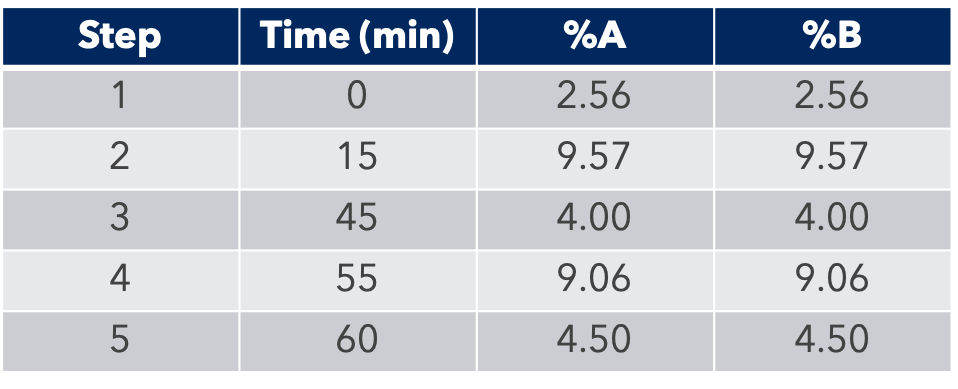
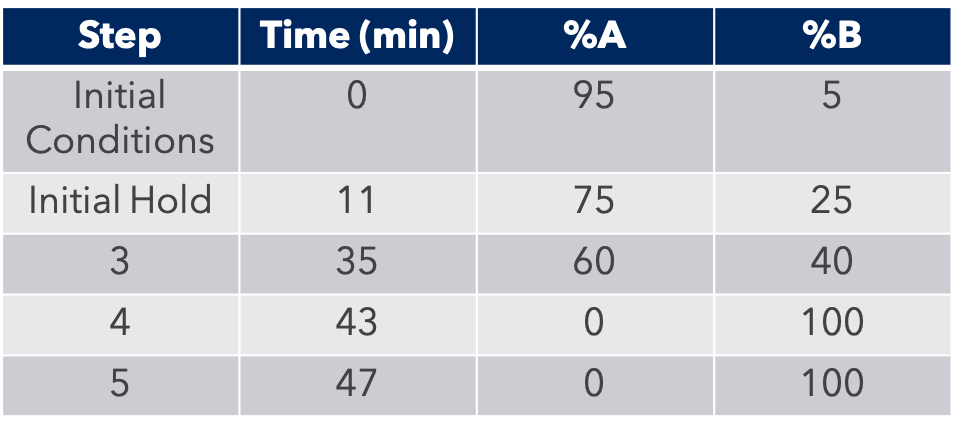
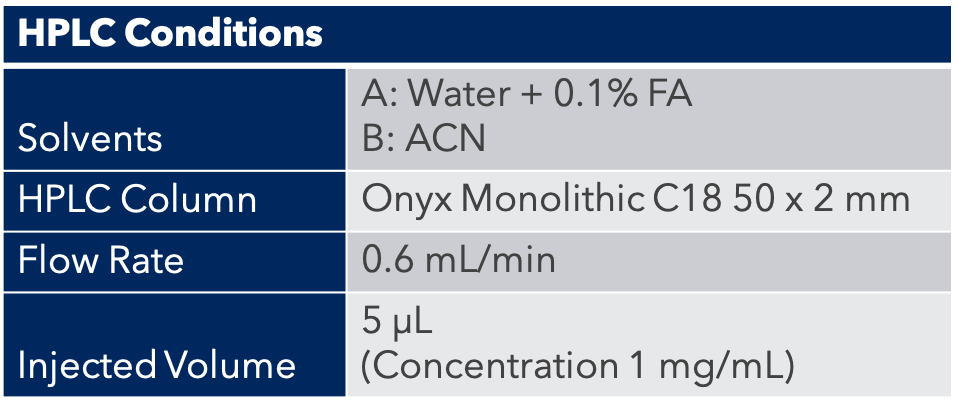
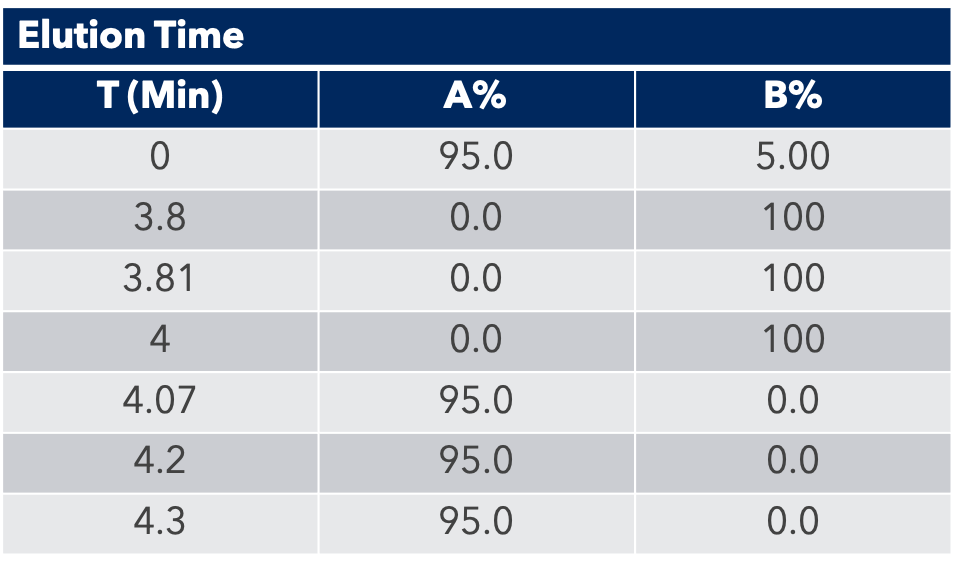
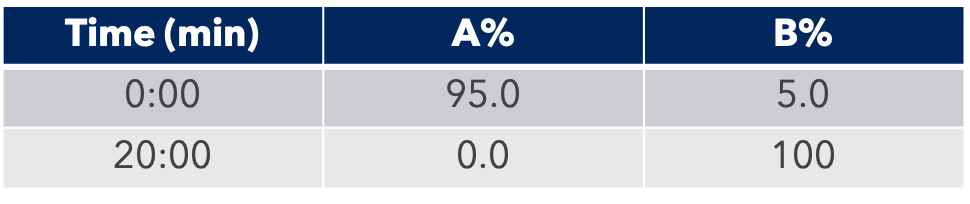
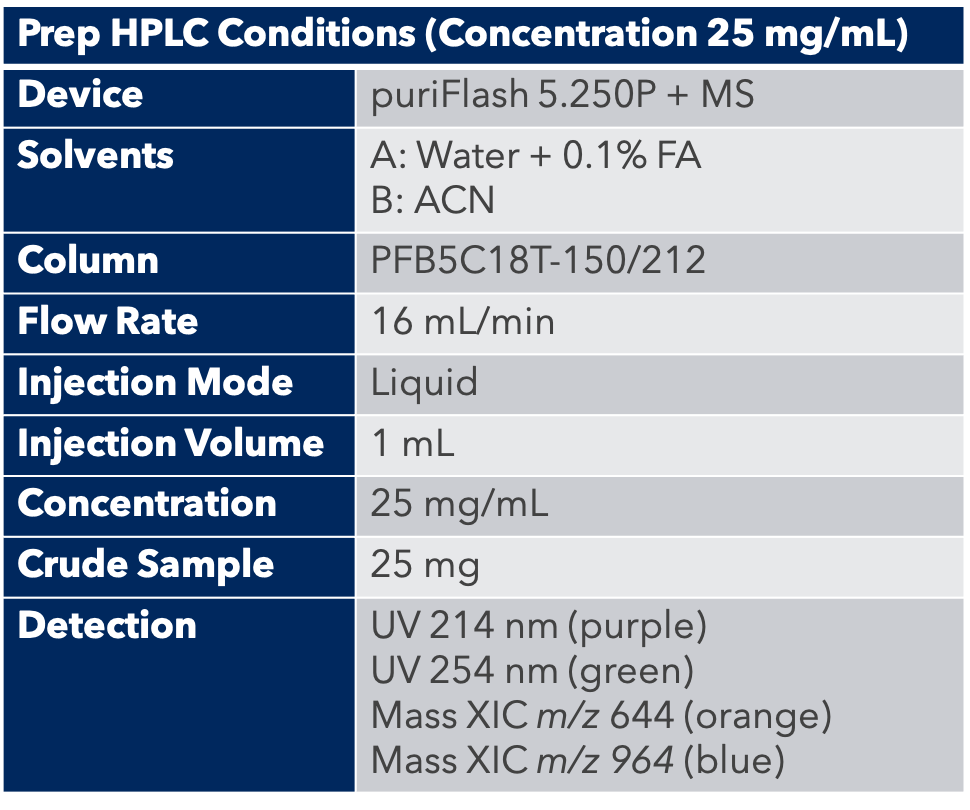
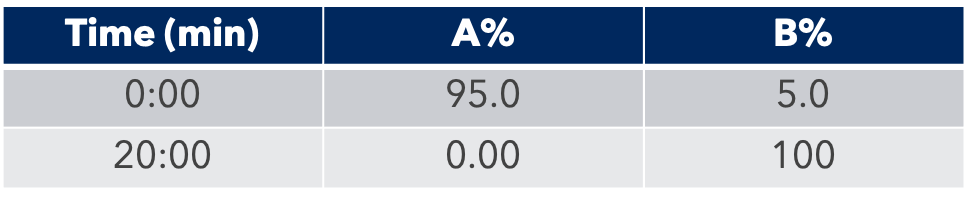
HPLC Conditions
Here are the results:
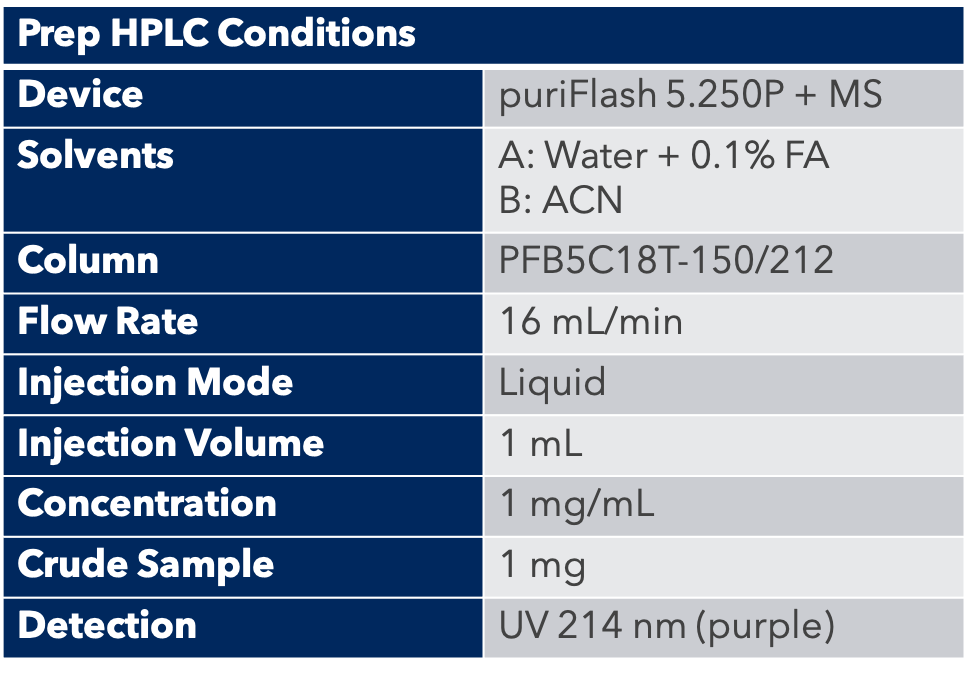
Transposition & Purification
A peptide column with a preparative column was used: PFB5C18T-150/212.
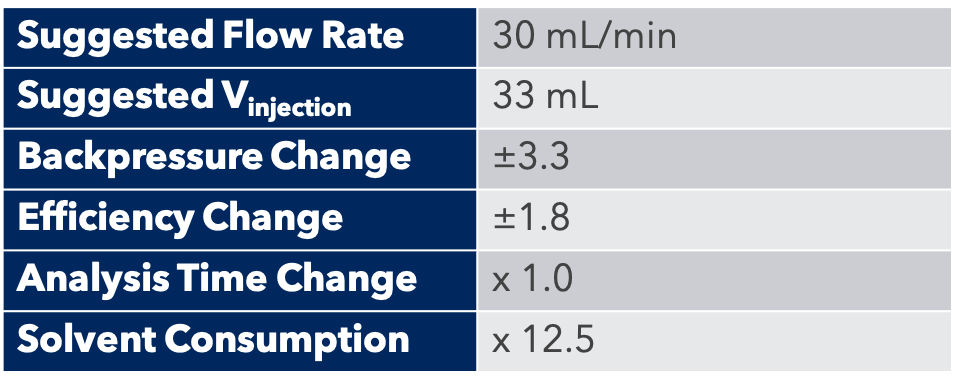
First, the analytical HPLC method was transposed to a preparative HPLC method.
Then, the concentration of the injected sample was increased to be able to purify the maximum amount of the product in a single run.
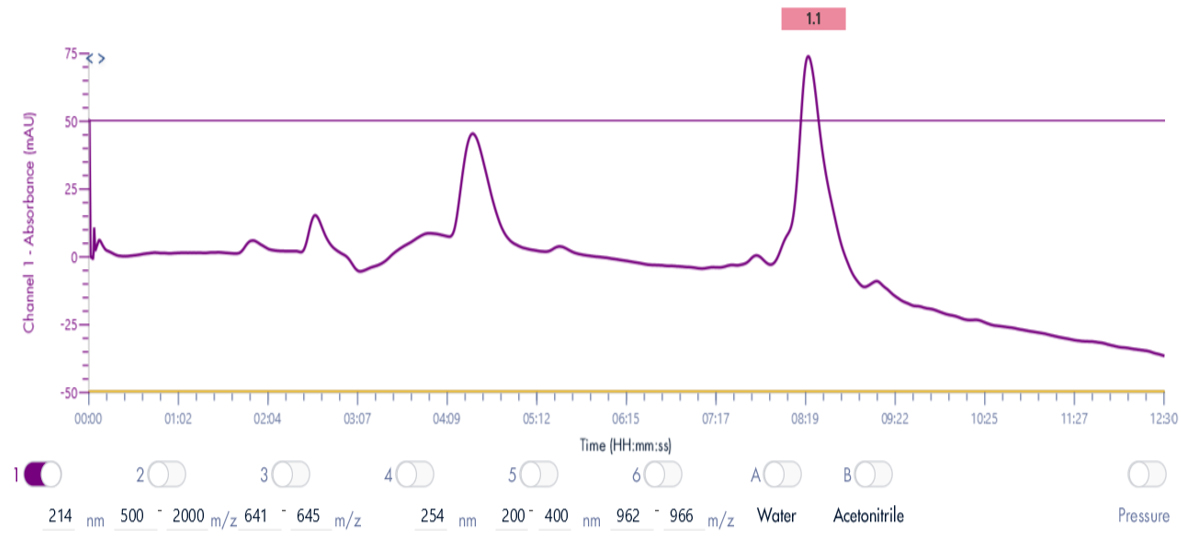
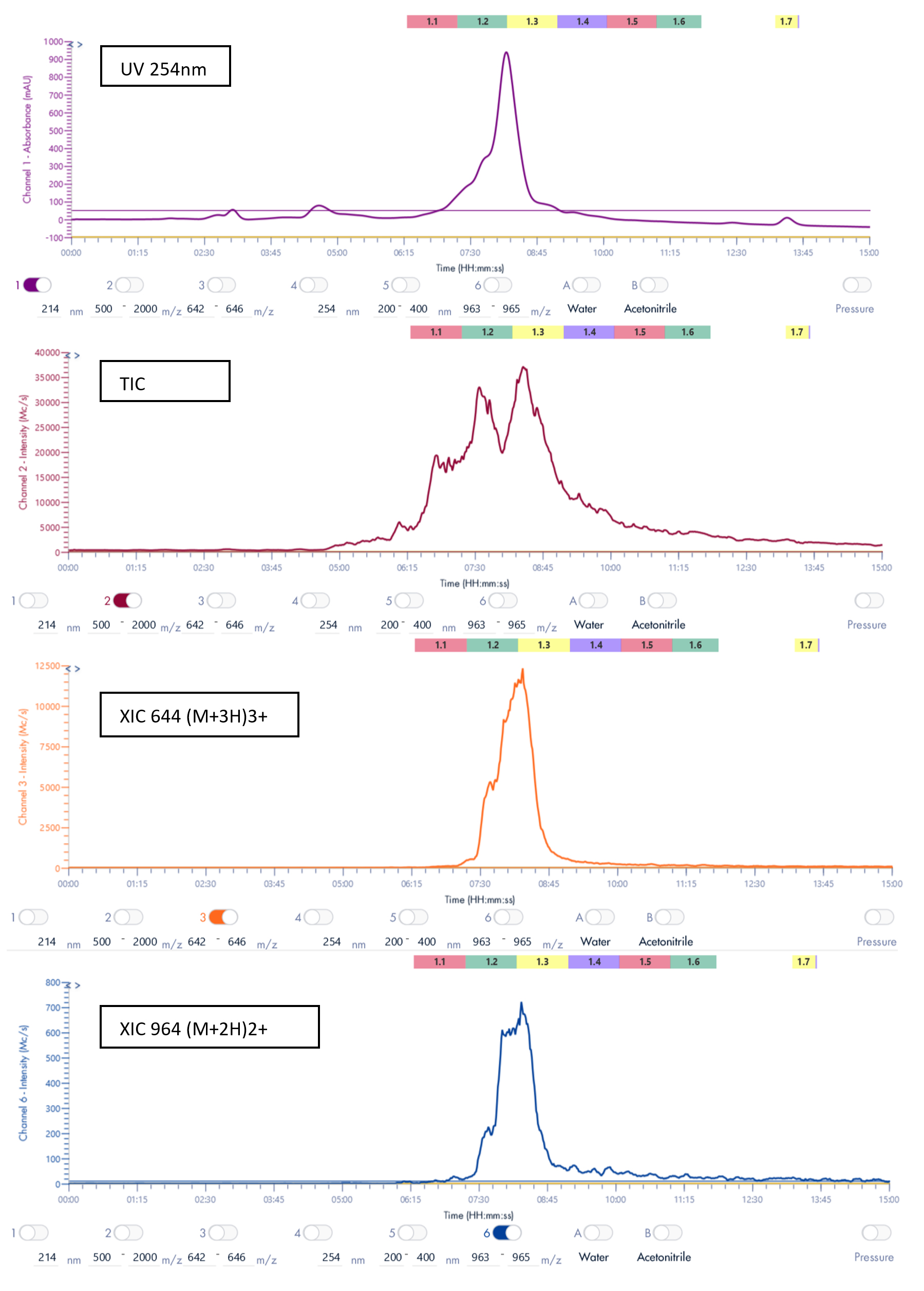 Thanks to the puriFlash 5.250P system coupled with the expression CMS, the product of interest was purified and collected based on its mass.
Thanks to the puriFlash 5.250P system coupled with the expression CMS, the product of interest was purified and collected based on its mass.
